Abstract [View PDF] [Read Full Text]
Objective
To observe the safety and efficacy of 0.01% atropine eye drops in the prevention of myopia onset in schoolchildren.
Methods
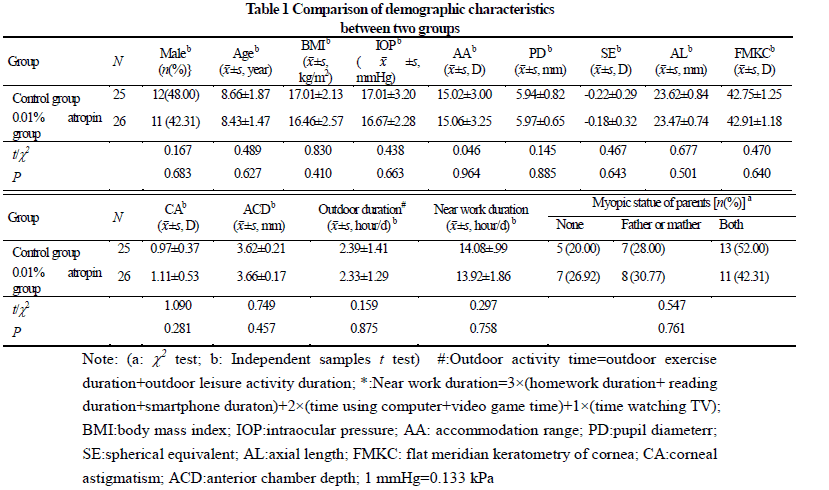
A randomized double-blind controlled study was conducted.Sixty Chinese Han children (60 eyes) with binocular spherical equivalent (SE) between + 0.50 D and -0.75 D (pre-myopia) by cycloplegic autorefraction treated in The First Affiliated Hospital of Zhengzhou University were enrolled from July to October 2020.Aged 6-12 years old, the children were divided into 0.01% atropine group and control group according to a random number table, with 30 cases (30 eyes) in each group.The children were given one drop of 0.01% atropine or placebo eye drops in both eyes once a night.The SE, axial length (AL), accommodative amplitude and pupil diameter were compared before and after 3-month, 6-month of treatment between the two groups.Discomforts were recorded.This study adhered to the Declaration of Helsinki.The study protocol was approved by an Ethics Committee of The First Affiliated Hospital of Zhengzhou University (No.2020-KY-286). Written informed consent was obtained from guardian of each subject.
Results
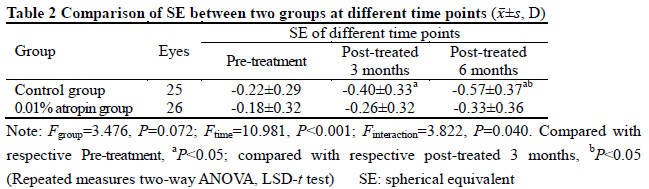
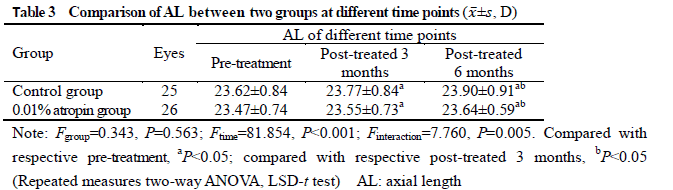
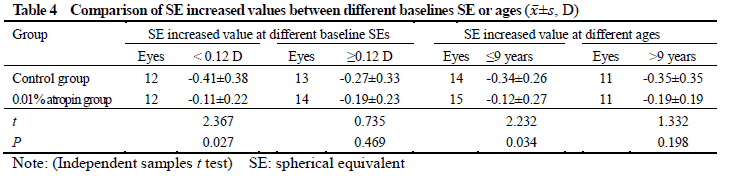
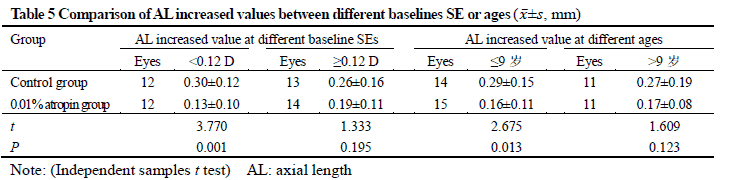
After treatment, 26 and 25 subjects completed the 6-month follow-up in 0.01% atropine group and control group, respectively, among which 3 subjects in 0.01% atropine group accounting for 11.5% and 9 in control group accounting for 36.0% developed myopia, showing a statistically significant difference (χ2=4.238, P=0.040). There were significant differences in the overall comparison of SE and AL at different time points between before and after treatment (Ftime=10.981, 81.854; both at P<0.001). At 3 and 6 months after treatment, there were significant increases in the SE and AL of control group and AL of 0.01% atropine group compared with respective baseline values (all at P<0.05). There was no significant difference in SE at 3 and 6 months after treatment compared with baseline SE in 0.01% atropine group (both at P>0.05). At 6 months after treatment, the change in SE in 0.01% atropine group was (-0.15±0.26)D, which was significantly less than (-0.34±0.35)D in control group, and the change in AL in 0.01% atropine group was (0.17±0.11)mm, Which was significantly shorter than (0.28±0.14)mm in control group, with significant differences between them (t=2.207, P=0.032; t=3.127, P=0.003). There were significant differences in pupil diameter at different time points between before and after treatment (Ftime=2.263, P=0.032). At 3 and 6 months after treatment, the pupil diameter was increased in comparison with baseline in 0.01% atropine group (both at P<0.05). There were significant differences in accommodative amplitude at different time points between before and after treatment in the two groups (Fgroup=0.882, P=0.042; Ftime=0.337, P=0.033). The accommodative amplitude at 3 and 6 months after treatment were decreased in comparison with baseline in 0.01% atropine group and control group at corresponding time points (all at P<0.05). Within a month after treatment, photophobia in bright sunlight occurred in 5 cases in 0.01% atropine group, accounting for 16.7%(5/30), and 2 cases in control group, accounting for 6.7%(2/30), showing no significant difference (χ2=0.647, P=0.421). No near-vision blur and other uncomfortable symptoms was found in the two groups.
Conclusions
After 6-month application of 0.01% atropine eye drops, the prevalence of myopia in pre-myopia schoolchildren decreases and the changing rate of SE and AL slows down.The accommodative amplitude is slightly reduced and pupil diameter is slightly increased, with no obvious effects on study and life.
Key words:
Figures and tables


Contributor Information
Department of Ophthalmology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450000, China
Department of Ophthalmology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450000, China
Department of Ophthalmology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450000, China
Department of Ophthalmology, The Second Affiliated Hospital of Zhengzhou University, Zhengzhou 450000, China
Department of Ophthalmology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450000, China
Department of Ophthalmology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450000, China
Department of Ophthalmology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450000, China
Department of Ophthalmology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450000, China
Henan Provincial People’s Hospital, Henan Eye Hospital, Henan Eye Institute, Zhengzhou 450003, China
Department of Ophthalmology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450000, China