Authors: Li Chunchun1, Tang Yuan2, Chen Zhangyan1, Wang Aisun1, Huang Xiaoqiong1, Chen Yanyan2, Qu Jia1
1Ophthalmology and Optometry, Eye Hospital of Wenzhou Medical University, Wenzhou 325000, China; 2School of Optometry and Ophthalmology, Wenzhou Medical University, Wenzhou 325000, China
Corresponding author: Chen Yanyan, Email: cyy@mail.eye.ac.cn;Qu Jia, E-mail:jqu@wz.zj.cn
[Abstract] Objective To evaluate the aerosol density (PM2.5, PM10.0 and aerosol particle number) formation in non-contact “air-puff” tonometry and provide suggestions for medical workers to take appropriate daily protection during the prevalence of 2019-nCoV. Methods A cross-sectional study was carried out in this study. Thirty healthy subjects were enrolled on February 22, 2020 at Eye Hospital of Wenzhou Medical University. The intraocular pressure (IOP) was measured by non-contact “air-puff” tonometer in the ophthalmic consulting room and the hall with or without masks. PM2.5, PM10.0 and aerosol particle number were recorded by air quality detector. The cumulative effects of IOP measurement, PM2.5, PM10.0 and aerosol particle number were analyzed, and the aerosol density of subjects with and without masks was compared. Results The PM2.5, PM10.0 and aerosol particle number produced by the non-contact “air-puff” tonometry and increased with the increase of spray times. The IOP curves of 60 eyes of 30 subjects were measured respectively in two environments of the ophthalmic consulting room and the hall. It was found that PM2.5, PM10.0 and aerosol particle number fluctuated and increased with the increase of IOP measurement person times, showing cumulative effect, and the accumulation speed of aerosol density in hall was faster than that in the ophthalmic consulting room. The density of PM2.5 and PM10.0 produced without masks were (53.417±2.306) and (85.350±3.488) μg/m3, which were higher than those of (50.567±0.862) and (80.617±1.463) μg/m3 with masks. The differences were statistically significant (P=0.028, 0.019). Conclusions Aerosol can be produced by non-contact “air-puff” tonometer spraying, and it fluctuates and increases with the increase of spraying times, showing a cumulative effect. The aerosol accumulation is higher in the hall with insufficient air circulation. And more aerosol can be produced without masks.
[Key words] Tonometry, Non-contact “air-puff”; Aerosol; Infection; Transmission; Corona virus disease-19
Funding program: Scientific research fund of national health and Family Planning Commission — key project of Zhejiang Province major medical and health science and technology plan (WKJ-ZJ-1727); Wenzhou Municipal City Emergency Research Grant for Prevention of 2019-nCoV (ZY 2020001).
DOI:10.3760/cma.j.issn.115989-20200226-00112
The 2019 novel coronavirus (2019-nCoV) is a new pathogen causing the outbreak of coronavirus disease-19 (COVID-19). It has the transmission between human and human beings [1], and is highly infectious, threatening public health of China and all over the word seriously. COVID-19 is mainly transmitted by respiratory droplets and close contact. When it is exposed to high concentration aerosol for a long time in a relatively closed environment, it may be transmitted by aerosol [2]. The ocular surface, oral mucosa and nasal mucosa are mucosa tissue. 2019-nCoV may contaminate the conjunctival epithelium, cause eye complications, and lead to respiratory tract infection [3]. At present, several cases of COVID-19 with conjunctivitis and conjunctivitis as first symptom have been reported [4-5]. Studies have shown that human tears contain more viruses, such as hepatitis B virus (HBV), viral hepatitis C (HCV), human immunodeficiency virus (HIV), herpes simplex virus, Epstein-Barr virus (EBV), mumps virus, measles virus [6-12], etc. Although 2019-nCoV has not been confirmed to exist in tears, a large number of previous studies have confirmed that tears contain severe acute respiratory syndrome (SARS) virus (SARS-coronavirus, SARS-CoV) [13] and Middle East respiratory syndrome (MERS) virus (MERS-coronavirus, MERS-CoV) with a highly similar genome to 2019-nCoV) [14]. Currently, several studies have confirmed that 2019-nCoV and SARS-nCoV share ACE2 receptor [15-16], while some studies have found that both conjunctiva and cornea express ACE2 receptor [17], the expression and distribution of ACE2 receptor are closely related to the transmission route and clinical symptoms of 2019-nCoV [18], and the ocular surface is easily the target organ of 2019-nCoV when exposed to the environment. Therefore, the transmission of 2019-nCoV via ocular surface route during the COVID-19 should not be ignored. In this study, we observed the dynamic changes of aerosol density in the use of non-contact “air-puff” tonometer, which is commonly used in ophthalmology clinical work, to provide reference and suggestions for ophthalmologists to do a good job in daily protection during the COVID-19.
1 Information and methods
1.1 General information
30 healthy subjects were enrolled on February 22, 2020 at Eye Hospital of Wenzhou Medical University, including 19 males and 11 females, aged 31-54 years. Inclusion criteria: ①no organic eye diseases and problems affecting vision; ②no infectious diseases; ③able to cooperate with ophthalmic examination; ④good compliance. Exclusion criteria: abnormal IOP. All the subjects knew the method and purpose of the study and signed the informed consent voluntarily before entering the experimental queue. This study was reviewed and approved by the ethics committee of Eye Hospital of Wenzhou Medical University (2020-018-k-16).
1.2 Methods
1.2.1 IOP measurement method: the change of aerosol was measured by non-contact “air-puff” tonometer (TX-20 automatic non-contact tonometer, Canon, Japan). The measurement was carried out in the ophthalmic consulting room and the hall. The non-contact “air-puff” tonometer was placed in the ophthalmology consulting room and the hall respectively for 60 eyes of 30 subjects for IOP measurement. Each subject’s both eyes were measured with or without masks. The changes of PM2.5, PM10.0 and aerosol particle number at the moment of IOP measurement were observed. During IOP measurement, each subject takes the sitting position, wears the mask, keeps quiet, fixes the jaw on the tonometer bracket, looks at the light source in the lens with both eyes, opens the eyes naturally and exposes the cornea. IOP was measured 3 times and the mean value was taken; then each subject takes off the mask and measures again. All measurements were made in the order of right eye first and then left eye.
1.2.2 Aerosol measurement method: Aerosol are measured by air quality detector (1000S+air quality detector, Temtop, USA). The detector was initially placed at the ventilation place for 24 hours for calibration. During the detection, the air quality detector was placed at the midpoint of the line between the tonometer lens and the mandible bracket. After IOP measurement, the aerosol density at the detection point were recorded.
1.2.3 Evaluation index: (1) PM2.5: the unit volume of aerodynamics in unit volume environment is≤2.5 μg particles. (2) PM10.0: the unit volume of aerodynamics in unit volume environment is<10.0 μg particles, also known as inhalable particulate matter. (3) Aerosol particle number: the number of individual particles per unit volume of air.
1.2.4 Standardization of testing site: one ophthalmic consulting room and one hall should be selected. The floor, working tables and some other surface of the ophthalmic consulting room and the hall should be wiped and ventilated for 30min before testing. The ophthalmic consulting room’s volume is 4.35m × 3.00m × 2.20m, and the hall’s volume is over 100m2.
1.3 Statistical methods
Epidata 3.1 was used to establish a database for parallel double entry, and at the same time, a verification file is established for computer logic verification. SPSS 25.0 was used for statistical analysis. In this study, the data of measurement indicators are normal distribution by Shapiro-Wilk test, and the variance between groups is homogeneous by Levene test. Two levels of repeated measurement were used to study the design. The paired t-test was used to compare the differences of PM and aerosol particle density measured by different instruments in the same group of subjects, P < 0.05 was statistically significant. The curve of aerosol density was drawn under the condition of continuous measurement of different measurement person times and different spray times of tonometer.
2 Results
2.1 Relationship between the times of tonometer spray and aerosol density
IOP was measured by one subject in the ophthalmology consulting room, IOP of both eyes was measured 3 times continuously. After six times of spray, PM2.5, PM10.0 and aerosol particle number all frustrated and increased with the increase of spray times of non-contact “air-puff” tonometer (Figure 1).

Figure 1 Relationship of tonometer spray times and aerosol density
2.2 Cumulative effect of IOP measurement person times and aerosol particle number
After IOP measurement, PM2.5, PM10.0 and aerosol particle number frustrated and increased with the increase of IOP measurement person times, showing a cumulative effect. And the accumulation speed of PM2.5, PM10.0 and aerosol particle number measured in the hall was faster than that in the ophthalmology consulting room (Figure 2-4).

Figure 2 Cumulative effect of PM2.5 upon the person-times of tonometry

Figure 3 Cumulative effect of PM10.0 upon the person-times of tonometry
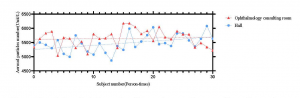
Figure 4 Cumulative effect of aerosol particle number upon the person-times of tonometry
2.3 Comparison of aerosol between subjects with and without masks
IOP was measured in subjects with and without masks. The density of PM2.5 and PM10.0 of subjects without masks was higher than that of subjects with masks (t = -2.836, P = 0.028; t = -3.066, P = 0.019). The density of aerosol particle number of subjects without masks was higher than that without masks, but the difference was not statistically significant (t = -1.687, P = 0.123) (Table 1).
Table 1 Comparison of aerosol and PM density between mask and non-mask (Mean±SD)
| Groups | Density of different kinds of aerosol | |||
| PM2.5(μg/m3) | PM10.0(μg/m3) | Aerosol particle number(Unit/L) | ||
| Mask | 50.567±0.862 | 80.617±1.463 | 7275.600±317.329 | |
| Non-mask | 53.417±2.306 | 85.350±3.488 | 7863.290±723.102 | |
| T value | -2.836 | -3.066 | -1.687 | |
| P value | 0.028 | 0.019 | 0.123 | |
3 Discussion
Aerosol is a kind of colloidal dispersion system which is composed of solid or liquid particles dispersed and suspended in the gas medium. The particle diameter is 0.01-10.0 μm [19]. The pathogenic microorganisms include virus, bacteria and fungi. Aerosols do not settle due to gravity, but can be suspended in the air or settled on the surface of the object [20]. Therefore, during the COVID-19, the virus is likely to form part of the aerosol particles. It is found that there is a significant correlation between 5-7 μ m particles and microbial pollution [21]. As a common ophthalmic examination equipment, non-contact “air-puff” tonometer uses the principle of gas pulse to spray gas to the central surface of cornea to measure the change of IOP. In 1991, Britt et al. Photographed a non-contact “air-puff” tonometer to measure IOP. At the moment of IOP measurement, the tear film on the ocular surface broke and splashed under the impact of air pressure, forming a large number of aerosol particle number, which continued to increase with the IOP spray times [22]. Therefore, if 2019-nCoV adheres to aerosol, it will form virus aerosol, which is very likely to lead to 2019 n-CoV propagation (Figure 5). But for a long time, there is a lack of knowledge about aerosol borne infection caused by medical equipment.
The results show that PM2.5, PM10.0 and aerosol particle number frustrate and increase with the increase of spray times and measurement times of non-contact “air-puff” tonometer. Because subjects’ breath can also produce a small amount of aerosol during the test, our experiment found that if subjects does not wear a mask, the aerosol density produced is higher than when wearing masks. In this study, it was also found that aerosol particles disappear rapidly with the air circulation. In the test, it was found that the accumulation speed of aerosol density in the hall was faster than that in the ophthalmology consulting room, and the hall was spacious because people were not allowed to walk, so the aerosol was easy to accumulate in local areas; The “one in one out” mode is adopted in the ophthalmology consulting room during the measurement. The air flow generated by the door opening and closing reduces the indoor aerosol density. Therefore, during COVID-19, the following suggestions are put forward for the correct use of non-contact “air-puff” tonometer commonly used in ophthalmic diagnosis and treatment to prevent 2019-nCoV iatrogenic cross infection.
3.1 Ventilation of ophthalmology consulting room
From figure 1-4 of this study, it can be seen that the aerosol density in the air frustrates and increases with the increase of spray times or measurement times of non-contact “air-puff” tonometer in a certain space, and there is a cumulative effect. Therefore, the non-contact “air-puff” tonometer should be placed in a relatively ventilated place to increase the air circulation and exchange. And the hospital should appropriately extend the interval time of measurement, especially reduce the concentration of personnel in the ophthalmology consulting room, follow the principle of “one doctor, one patient, one diagnosis room” and “treatment in different periods”, and try to shorten the stay time of patients in the hospital, so as to reduce the generation of aerosol particles and Cumulative.
3.2 Cleaning and disinfection of object surface of ophthalmology consulting room
Because aerosol particles are easy to settle on the surface of the table and objects, medical staff and patients may be infected after contact. Therefore, strict cleaning and disinfection should be paid attention to during COVID-19. It is suggested that “one person, one use, one disinfection”. After each subject uses it, medical staff should spray the lens with 75% alcohol immediately, wipe the mandible, forehead and armrest of the tonometer. During COVID-19, instrument disinfection must be carried out strictly, that is, after the operation of the instrument every day, the surface, base and both sides of the isolation plate of the tonometer should be wiped with 1000mg/L chlorine containing disinfectant, and then wiped with clean water. Medical staff should wipe the surface of mandible bracket, frontal bracket and eyepiece protective cover with 75% alcohol cotton ball, screw off the eyepiece protective cover during final disinfection treatment, spray with 75% alcohol or ether to wipe the lens, and screw on the protective cover after volatilization. The air in the ophthalmology consulting room is sterilized by dynamic sterilizer or ultraviolet for 30 minutes in the morning, middle and evening every day.
3.3 Self-protection of medical staff and patients
According to table 1 of this study, PM2.5, PM10.0 and aerosol particle number of subjects without masks were higher than that without masks. Therefore, in the clinical work of Ophthalmology, especially duringCOVID-19, it is suggested that the patients with IOP should wear disposable medical masks and try not to speak; During the operation of non-contact “air-puff” tonometer, medical staff must wear medical goggles or protective mask. And at the same time, medical staff need to wear medical surgical mask, medical cap and disposable medical gloves; During the use of the non-contact “air-puff” tonometer, a protective baffle should be placed between the medical staff and the patients. It is recommended to place a transparent isolation plate between the tonometer eyepiece and the forehead bracket, such as a self-made X-film, to prevent the spray of aerosol particles; In the ophthalmology consulting room, “one doctor, one patient, one consulting room” should be implemented; The waiting patients should keep a safe distance of at least 1m to reduce the spread of droplets and aerosols in the air.
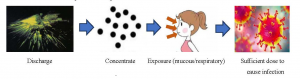
Figure 5 Operative principle of non-contact “air-puff” tonometer to cause COVID-19 via aerosol
Thank you Chen Yuejie, Guo Yuanyuan and Huang Susu for their help in data collection.
Conflict of interest: there is no conflict of interest in this study.
References
[1] Chan JFW, Y uan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novelcoronavirus indicating person-to-person transmission: a study of a familycluster[J/OL]. Lancet, 2020[2020-02-24]. http://www.sciencedirect.com/science/article/pii/S0140673620301549. DOI:10.1016/S0140-6736(20)30154-9.
[2] 国家卫生健康委办公厅,中医药局办公室. 新型冠状病毒肺炎诊疗方案(试行第六版修正版)[S/OL]. 2020-02-19[2020-02-24]. http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a 8326dd94d329df351d7da8aefc2.shtml.
[3] Lu CW, Liu XF, Jia ZF. 2019-nCoV transmission through the ocular surface must not be ignored[J/OL]. The Lancet, 2020[2020-02-24]. https://www.sciencedirect.com/science/article/pii/S0140673620303135. DOI:10.1016/S0140-6763(20) 30313-5.
[4] 叶娅, 宋艳萍, 闫明, 等. 新型冠状病毒肺炎合并结膜炎三例 [J/OL] . 中华实验眼科杂志,2020,38.2020-02-24[2020-02-25]. http://rs.yiigle.com/ yufabiao/1182653.htm. DOI:10.3760/cma.j.issn.2095-0160.2020.0006.
[5] 李雪杰, 汪明, 陈长征, 等. 伴发或首发病毒性结膜炎的新型冠状病毒感染下眼科医师 的防控策略[J/OL] . 中华实验眼科杂志, 2020, 38(00): E002-E002.2020-02-16[2020-02-25]. http://rs.yiigle.com/yufabiao/1181982. DOI:10.3760/cma.j.issn.2095- 0160. 2020.0002
[6] Komatsu H, Inui A, Sogo T, et al. Tears from children with chronic hepatitis B virus (HBV) infection are infectious vehicles of HBV transmission: experimental transmission of HBV by tears, using mice with chimeric human livers[J]. J Infect Dis, 2012, 206(4):478-485. DOI:10.1093/infdis/jis293.
[7] Pfaender S, Helfritz FA, Siddharta A, et al. Environmental stability and infectivity of hepatitis C virus (HCV) in different human body fluids[J]. Front Microbiol, 2018, 9:504. DOI:10.3389/fmicb.2018.00504.
[8] Han Y, Wu N, Zhu WJ, et al. Detection of HIV-1 viruses in tears of patients even under long-term HAART[J]. AIDS, 2011, 25(15): 1925-1927. DOI:10.1097/qad.0b013e32834b3578.
[9] Fukuda M, Deai T, Higaki S, et al. Presence of a large amount of herpes simplex virus genome in tear fluid of herpetic stromal keratitis and persistent epithelial defect patients[J]. Semin Ophthalmol, 2008, 23(4):217-220. DO:10.1080/088205308021 11366.
[10] Willoughby CE, Baker K, Kaye SB, et al. Epstein-Barr virus (types 1 and 2) in the tear film in Sjögren’s syndrome and HIV infection[J]. J Med Virol, 2002, 68(3): 378-383. DOI:10.1002/jmv.10214.
[11] Kalkan A, Ozden M, Yilmaz T, et al. A case of mumps conjunctivitis: detection of the virus RNA by nested PCR in tear sample[J]. Scand J Infect Dis, 2004, 36(9):697-700. DOI:10.1080/00365540410022648.
[12] Shinoda K, Kobayashi A, Higashide T, et al. Detection of measles virus genomic RNA in tear samples from a patient with measles keratitis[J]. Cornea, 2002, 21(6):610-612. DOI:10.1097/00003226-200208000-00017.
[13] Bonn D. SARS virus in tears?[J]. Lancet Infect Dis, 2004, 4(8):480.
[14] 吴清民, 汪明. 动物冠状病毒的研究进展[J]. 中国农业科技导报, 2003,5(4): 17-24.
[15] Zhou P, Y ang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin[J/OL]. Nature, 2020[2020-02-24]. https://www. nature.com/articles/s41586-020-2012-7. DOI:10. 1038/s41586-020-2012-7.
[16] Wan YS, Shang J, Graham R, et al. Receptor recognition by novel coronavirus from Wuhan: An analysis based on decade-long structural studies of SARS[J/OL]. J Virol, 2020[2020-02-24]. https://jvi.asm.org/content/early/2020/01/23/JVI.00127-20. DOI:10.1128/JVI.00127-20.
[17] 孙琰, 柳林, 潘欣, 等. SARS-CoV S240 蛋白与眼部 ACE2 受体作用机制的研究[J]. 国际眼科杂志, 2006, 6(4): 783-786. DOI: 10.3969/j.issn.1672- 5123. 2006.04.014.
[18] Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding[J]. Lancet, 2020[2020-02-24]. https://medicalxpress.com/news/2020-01-coronavirus-genetically-sars-human-infecting.html. DOI: 10.1016/S0140-6736(20)30251-8.
[19] 上海社会科学院青年学术交流中心编. 理论热点大碰撞[M]. 上海: 上海人民出版社, 2015: 42.
[20]《环境科学大辞典》编辑委员会. 环境科学大辞典[M]. 北京: 中国环境科学出版社, 1991: 496.
[21] Mirhoseini SH, Nikaeen M, Khanahmd H, et al. Monitoring of airborne bacteria and aerosols in different wards of hospitals-particle counting usefulness in investigation of airborne bacteria[J]. Ann Agric Environ Med, 2015, 22(4): 670-673.DOI: 10.5604/12321966.1185772.
[22] Britt JM, Clifton BC, Barnebey HS, et al. Microaerosol formation in noncontact “air-puff” tonometry[J]. Arch Ophthalmol, 1991, 109(2): 225-228. DOI:10.1001/archopht.1991.01080020071046.