·Clinical Research·
Long-term influence of donor graft thickness and graft size on corneal endothelial cell density of Descemet’s stripping automated endothelial keratoplasty
Gu Shaofeng, Peng Rongmei, Xiao Gege, Feng Yun, Hong Jing
Department of Ophthalmology, Beijing Key Laboratory of Restoration of Damaged Ocular Nerve, Peking University Third Hospital, Beijing100191, China
Corresponding author: Hong Jing, Email: hongjing196401@163.com
[Abstract] [View PDF in English] [View PDF in Chinese] [Read Full Text]
Objective To characterize the long-term influence of donor central graft thickness (CGT) and graft size on corneal endothelial cell density (ECD) after Descemet’s stripping automated endothelial keratoplasty (DSAEK).
Methods An observational case series study was conducted. One hundred and forty-four eyes of 134 patients who underwent DSAEK at the Peking University Third Hospital between January 2013 and December 2017 with at least 24-month follow-up were enrolled. Preoperative donor ECD was evaluated by eye bank specular microscopy, and postoperative ECD was determined by in vivo confocal microscopy at 1, 3, 6, 12, and 24 months after surgery. Corneal endothelial cell loss was calculated according to the pre-and postoperative ECDs. Donor CGT was measured by anterior segment optical coherence tomography. According to the 3-month postoperative donor CGT, patients were divided into the thinner graft group (45 eyes with donor CGT < 100 μm), the medium thick graft group (66 eyes with donor CGT ≥ 100~< 150 μm), and the thicker graft group (33 eyes with donor CGT ≥ 150 μm). According to donor trephination size, the patients were divided into the smaller graft group (31 eyes with donor trephination size ≥ 7~< 8 mm) and the larger graft group (113 eyes with donor trephination size ≥ 8~< 9 mm). The postoperative donor CGT and endothelial cell loss at the different observation time points were compared, and the relationnships between the 24-month postoperative ECD and the preoperative donor ECD, the donor CGT, and the donor graft size were analyzed.
Results The donor CGTs were 129.0 (90.8, 160.8), 115.5 (93.0, 146.0), 115.5 (89.0, 151.0), 112.5 (94.3, 146.8), and 114.0 (89.0, 144.5) μm at 1, 3, 6, 12 and 24 months after surgery, respectively, showing a statistically significant difference (H = 37.369, P < 0.001). There was a significant difference in postoperative donor CGT between 1-month and 3-month (P < 0.001) patients. Spearman’s correlation analysis showed that the 24-month postoperative ECD was positively correlated with the preoperative donor ECD (rs = 0.783, P < 0.001), which was not associated with donor graft size and donor CGT (rs = 0.141, P = 0.093; rs = -0.044, P = 0.600), respectively. There was no significant difference in the rate of endothelial cell loss among the three different donor CGT groups and between the two different donor size groups at any postoperative observation time point (all, P > 0.05).
Conclusions Postoperative ECD was correlated with the preoperative ECD of the donor graft. The lower long-term rate of endothelial cell loss after DSAEK was associated with the thinner and larger diameter of the donor graft.
[Key words] Cornea; Endothelial cells; Corneal endothelial cell loss; Corneal endothelial cell density; Descemet’s stripping automated endothelial keratoplasty; Donor graft thickness; Donor trephination size
Fund program: National Natural Science Foundation of China (81970768)
DOI: 10.3760/cma.j.cn115989-20200408-00247
Descemet’s stripping automated keratoplasty (DSAEK), as one of the most common methods for the treatment of corneal endothelial diseases, has replaced traditional penetrating keratoplasty because of its advantages of less rejection, shorter learning curve, less risk of surgery, and rapid recovery of postoperative vision, and has facilitated the extensive development of corneal endothelial transplantation worldwide1. Some studies found that a thin donor corneal endothelial graft led to better postoperative visual recovery and a low rate of endothelial cell loss2-3. Therefore, based on traditional DSAEK, thin graft surgical protocols, such as ultra-thin DSAEK4, have been developed. However, its use is still controversial. Numerous studies have reported that the thickness of the donor graft was not correlated with postoperative vision and corneal endothelial density (ECD)5-7, and some studies reported that thin donor graft increased the loss of corneal endothelial cells8. The anatomical characteristics of eyes in Chinese patients differ from those in Western countries. A previous study reported that donor grafts in Western countries were usually larger, with a diameter of ≥ 8.5 mm for DSAEK9, but the grafts in China were smaller, with a diameter of ≤ 8.0 mm. However, the effect of donor graft thickness and size on postoperative ECD has not been reported in Chinese patients. This study therefore aimed to characterize the influence of donor graft thickness and size on the 24-month postoperative ECDs in China, to provide a possible basis for performing small graft DSAEKs.
1 Patients and Methods
1.1 Participants
This study was a retrospective case series enrolling 144 eyes of 134 patients who underwent DSAEK surgery for corneal endothelial decompensation between January 2013 and December 2017 at the Ophthalmic Research Center Affiliated with Peking University Third Hospital. The mean age was 52.2 ± 20.2 years (range: 4−88 years) with 65 males (73 eyes) and 69 females (71 eyes). There were 74 right eyes and 70 left eyes in the study. All patients completed a 24-month follow-up with complete data, and with a mean preoperative intraocular pressure of 15.4 ± 5.3 mmHg (1 mmHg = 0.133 kPa). The causes of corneal endothelial decompensated ion balance included 14 cases of Fuchs corneal endothelial dystrophy, 11 cases of other types of corneal endothelial dystrophy, 54 cases after cataract extraction, 19 cases after anti-glaucoma surgery, seven cases after vitrectomy, 20 cases after corneal transplantation, six cases of eye trauma, and 13 cases from other causes. The patients were divided into three groups according to the 3-month postoperative donor central graft thickness (CGT), because it was stable at 3 months after surgery. The thinner graft group was CGT < 100 μm, n = 45; the medium-thick graft group was CGT ≥ 100~< 150 μm, n = 66; the thicker graft group was CGT ≥ 150 μm, n = 33. According to the donor trephination size, the patients were divided into a smaller graft group (31 eyes with donor graft size ≥ 7−< 8 mm) and a larger graft group (113 eyes with donor graft size ≥ 8−< 9 mm). This research followed the tenets of the Declaration of Helsinki and was approved by the Institutions Ethics Committee of Peking University Third Hospital (approval number: IRB00006761-2008025). All patients signed informed consents before surgery.
1.2 Methods
1.2.1 Donor ECD examinations The donor corneal grafts were preserved at 4°C in K-Sol medium (Cilco, Huntington, WV, USA), and an Eye Bank specular microscope (HAI EB-3000XYZ; HAI Laboratories, Lexington, MA, USA) was used to photograph images of the central donor endothelium. The clear image of the endothelial layer was selected and the donor ECD was evaluated by device intrinsic software. Apices of 50~100 cells from the endothelial images for each cornea were counted and analyzed.
1.2.2 ECD examinations by in vivo confocal microscopy In vivo corneal laser scanning confocal microscopy (HRT3/RCM; Heidelberg Engineering, Dossenheim, Germany) was used to measure the ECD at 1, 3, 6, 12, and 24 months after surgery. Before examinations, a drop of topical anesthetic (4 g/L Oxybuprocaine hydrochloride; Santen Pharmaceutical, Osaka, Japan) was instilled in the lower conjunctival fornix. After applying a drop of gel on the front surface of the microscope lens, a disposable sterile cap was mounted on the holder to cover the microscope lens. The height of the lens was adjusted so that the cap was slightly in contact with the central section of the cornea, and each layer of the cornea was examined. The number of endothelial cells was counted manually, and the postoperative ECD was determined as the number of cells/mm2 using the proprietary software within the corneal confocal microscope. The rate of endothelial cell loss was calculated according to the postoperative ECD at different time points and the corresponding preoperative donor ECD. The rate of endothelial cell loss = (the preoperative donor ECD – the postoperative ECD) / the preoperative donor ECD.
1.2.3 Pachymetry of central corneal thickness and CGT The preoperative recipient central corneal thickness (CCT) and postoperative CCT were measured using anterior-segment optical coherence tomography (AS-OCT; Visante, Carl Zeiss Meditec, Dublin, CA, USA). The same operator adjusted the software system to position the vertex at the center of the AS-OCT image. More than three horizontal scans were performed, and the scan with the best quality was selected for measurement. The high-resolution corneal modules were used for measurements. The thickness was measured using software of the Visante AS-OCT system. The preoperative recipient CCT and postoperative CCT were measured with the caliper position at zero and recorded as the distance from the surface epithelium to the endothelium. The CGT was the distance between the high light reflective plane (i.e. the graft–host interface) and the endothelium.
1.3 Statistical analysis
Statistical analyses were performed using SPSS statistical software for Windows, version 21.0 (IBM, Armonk, NY, USA). Data were verified to be inconsistent with a normal distribution of continuous variables using the Shapiro–Wilk test and expressed as the M (Q1, Q3). The pre- and postoperative CCTs and the donor CGTs at each postoperative time point were compared using the Kruskal–Wallis H test, and the Wilcoxon test was used to compare the paired data. Spearman’s rank correlation analysis was used to evaluate the relationships between postoperative 24-month ECD and preoperative donor ECD, CGT, or graft size. The Kruskal–Wallis H test was performed to analyze the differences of ECD and endothelial cell loss rate among various time points and Mann–Whitney U test was used for post-hoc analysis. A two-tailed test was selected, and P < 0.05 was considered statistically significant.
2 Results
2.1 Comparison of pre-and postoperative CCTs, and donor CGTs after surgery
The preoperative CCT was 797.5 (722.5, 910.0) μm, and 641.5 (590.5, 730.0) μm at postoperative 1-month group, 619.5 (577.0, 691.5) μm at postoperative 3-month group, 625.0 (572.5, 699.0) μm at postoperative 6-month group, 627.0 (584.5, 692.5) μm at postoperative 12-month group, and 623.0 (578.0, 692.0) μm at postoperative 24-month group, showing a statistically significant difference among different time points (H = 259.008, P < 0.001). The CCT value was significantly lower at postoperative time points than that at preoperation (all, P < 0.05). The donor CGT was 129.0 (90.8, 160.8) at postoperative 1-month group, 115.5 (93.0, 146.0) at postoperative 3-month group, 115.5 (89.0, 151.0) at postoperative 6-month group, 112.5 (94.3, 146.8) at postoperative 12-month group, and 114.0 (89.0, 144.5) at postoperative 24-month group, showing a statistically significant difference among different time points (H = 37.369, P < 0.001), and the difference between between postoperative 1-month group and postoperative 3-month group was significantly different (P < 0.001). There was no significant difference in the donor CGTs between postoperative 3-month group and postoperative 6-month group, postoperative 3-month group and postoperative 12-month group, or postoperative 3-month group and postoperative 24-month group (all, P > 0.05).
2.2 The ECD and endothelial cell loss rate at different time points after surgery
The preoperative ECD of the donor was 2,455.9 (2,250.0, 2,872.3) cells/mm2. After surgery, there was a progressive decreased in the donor ECD over time and the endothelial cell loss rate was increased gradually (Table 1).

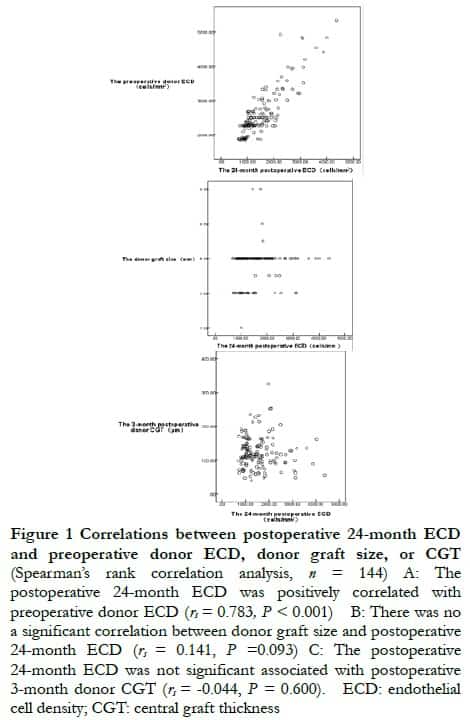
2.3 Correlation between postoperative 24-month ECD and preoperative donor ECD, donor graft size, or donor CGT
Spearman’s correlation analysis showed that the postoperative 24-month ECD was positively correlated with preoperative donor ECD (rs = 0.783, P < 0.001), and postoperative 24-month ECD was not associated with donor graft size, or postoperative 3-month donor CGT (rs = 0.141, P = 0.093; rs = –0.044, P = 0.600, respectively) (Figure 1).
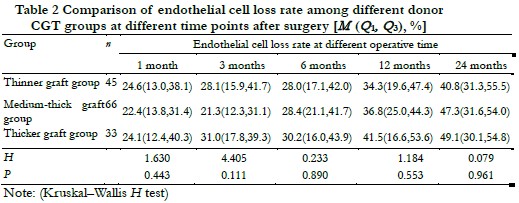
2.4 Comparison of the preoperative ECD and endothelial cell loss rate among different donor CGT groups
The donor CGT was 72.0 (62.0, 89.5) μm in the thinner graft group, 118.5 (111.8, 135.3) μm in the medium-thick graft group, and 171.0 (164.0, 209.0) μm in the thicker graft group. The preoperative donor ECD of the three groups were 2 499.8 (2 251.1, 3 033.0), 2 458.5 (2 250.0, 3 013.0), and 2 424.0 (2 254.9, 2 713.9) cells /mm2, and the difference of preoperative donor ECD was not statistically significant among different donor CGT groups (H = 0.368, P = 0.832). There was no significant difference in endothelial cell loss rate among different donor CGT groups at postoperative 1, 3, 6, 12 and 24 months (H = 1.630, 4.405, 0.233, 1.184, 0.079, all at P > 0.05) (Table 2).
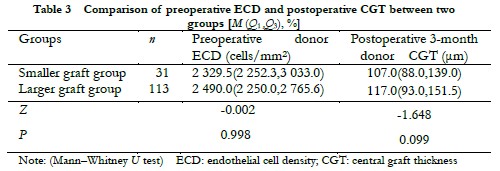
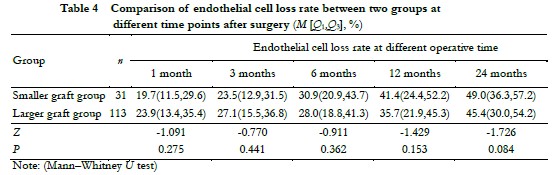
2.5 Comparison of preoperative donor ECD, postoperative donor CGT, and endothelial cell loss rate between different donor size groups
The diameter of the smaller graft group was 7.5 (7.5, 7.5) mm and 8.0 (8.0, 8.0) mm in the larger graft group. There was no significant difference in preoperative donor ECD and the 3-month postoperative donor CGT between the two groups (Z = -1.648 and -0.002, all, P > 0.05) (Table 3). The difference in the rate of endothelial cell loss was not significant between the two groups at each observation time point after surgery (all, P > 0.05) (Table 4).
3 Discussion
Previous studies reported that donor grafts might “swell” before DSAEK and presumed that this phenomenon was mainly due to removal of the corneal epithelium when the grafts were irrigated in a balanced salt solution. After surgery, donor implants could have a “deturgescence” effect and reduce the thickness of the donor graft10. In the current study, the CCT and the donor CGT decreased significantly at postoperative 1 and 3 months, then gradually stabilized after three months. The reason might be inflammation after surgery and the possibility that surgical injury of graft endothelial cells subsided at 3 months after DSAEK. The recovery of density and function of the endothelial cells might lead to the thickness of the CCT and CGT being stable. Di Pascuale et al.11 reported that donor graft thickness decreased after DSAEK, from 243 μm on the first day after surgery to 148 μm at the last follow-up, which suggested that donor grafts might have a “deturgescence” after DASEK. In the present study, the donor CGT decreased from 129.0 (90.8, 160.8) μm at 1 month to 115.5 (93.0, 146.0) μm at 3 months. The change in the donor graft thickness may therefore be important when preparing donor grafts by eye banks and evaluating surgical efficacy. If the thickness of the graft is more than 100 μm before surgery, it might become thinner after surgery and might result in an ultrathin DSAEK.
Based on the above analysis, we found that the donor CGT was stable at 3 months after DSAEK. We then analyzed possible correlations between the 24-month postoperative ECD and the preoperative donor ECD, donor graft size, and the 3-month postoperative donor CGT. The results showed that the 24-month postoperative ECD had a strong positive correlation with the preoperative donor ECD, but had no significant correlation with donor graft size and 3-month postoperative donor CGT. These results were consistent with previous studies. Lass et al.12 reported that the ECD at 3 years after DSAEK surgery was positively correlated with the preoperative donor ECD, but had no significant correlation with donor graft thickness. Lekhanont et al.13 also suggested that ECD at 5 years after DSAEK surgery was positively correlated with preoperative donor ECD, but not with the donor graft diameter.
In 2012, Busin et al.14 performed ultrathin DSAEK surgery with a donor graft thickness of less than 100 μm. Since then, many studies have reported that ultrathin DSAEK has the advantages of rapid vision recovery and small postoperative high order astigmatism15-16. However, the relationship between donor CGT and visual acuity recovery after DSAEK remains controversial17. In the current study, the rate of endothelial cell loss in the thicker graft group was higher than in the other two groups during 24 months of follow-up, except for postoperative 1-month, which was lower than the thinner graft group. The high rate of endothelial cell loss in the thinner graft group at postoperative 1-month might be related to the relatively complicated surgical procedure and difficulty in unfolding the lamella during surgery. The rate of endothelial cell loss in the thinner graft group was lower than in the other two groups between 6 and 24 months after surgery. Less inflammation and rejection due to less corneal stroma of the thin lamella in the thinner graft group might be the reason. The high rate of endothelial cell loss in the thicker graft group between 3 and 24 months might be related to the relative amount of stress on donor endothelial cells. When the lamella entered the anterior chamber, the double squeezing effect on graft endothelium from the corneal incision and the port of the Busin glide was more severe in the thicker graft group. Moreover, the thicker lamella might have more serious inflammation and rejection due to more corneal stroma. However, there was no significant difference in the rate of endothelial cell loss among the three groups at each observation time point. Therefore, the rate of endothelial cell loss might be lower in relatively thinner donor grafts in DSAEK patients.
We also determined the differences in preoperative donor ECDs and 3-month postoperative donor CGTs between the two different-size graft groups, but found no statistical significance between the two groups. The rate of endothelial cell loss in the smaller graft group was lower than in the larger graft group 1−3 months after DSAEK, so more injury of donor endothelial cells due to the squeezing effect of the corneal incision and the port of the Busin glide in the large graft might be the reason. The rate of endothelial cell loss in the large graft group was lower than in the smaller graft group between 6 and 24 months. This might be related to the larger graft containing more corneal endothelial cells and some damaged endothelial cells gradually recovering their function. However, there was no significant difference in the rate of endothelial cell loss between the two groups at each observation time point. Therefore, the rate of endothelial cell loss might be lower in relatively large-size donor grafts in DSAEK.
In summary, the results of the current study showed that donor CGT tended to be stable at 3 months in small graft DSAEK patients, which was consistent with the eye characteristics of Chinese patients. The 24-month postoperative ECD was positively correlated with preoperative donor ECD, but not with the donor CGT and graft size. The thinner and larger size of the donor graft, and the lower rate of endothelial cell loss after DSAEK, was more beneficial for long-term survival of the graft.
Confliction of interest None declared.
Author contribution statement Data collection and analysis were performed by Gu Shaofeng and Peng Rongmei. The first draft of the manuscript was written by Gu Shaofeng. Data interpretation and analysis were performed by Xiao Gege and Feng Yun. Writing, review, and funding acquisition were performed by Hong Jing.
References
[1] Peng RM, Hong J. Influence of different corneal diseases on visual recovery following Descemet stripping automated endothelial keratoplasty[J]. Chin J Exp Ophthalmol, 2014, 32(5):415-419. DOI: 10.3760/cma.j.issn.2095-0160.2014.05. 007.
[2] Neff KD, Biber JM, Holland EJ. Comparison of central corneal graft thickness to visual acuity outcomes in endothelial keratoplasty[J]. Cornea, 2011, 30(4): 388- 391. DOI: 10.1097/ICO.0b013e3181f236c6.
[3] Acar BT, Akdemir MO, Acar S. Visual acuity and endothelial cell density with respect to the graft thickness in Descemet’s stripping automated endothelial keratoplasty: one year results[J]. Int J Ophthalmol, 2014, 7(6):974-979. DOI: 10.3980/j.issn.2222-3959.2014.06.11.
[4] Busin M, Albé E. Does thickness matter: ultrathin Descemet stripping automated endothelial keratoplasty[J]. Curr Opin Ophthalmol, 2014, 25(4):312-318. DOI: 10.1097/ICU.0000000000000071.
[5] Shinton AJ, Tsatsos M, Konstantopoulos A, et al. Impact of graft thickness on visual acuity after Descemet’s stripping endothelial keratoplasty[J]. Br J Ophthalmol, 2012, 96(2):246-249. DOI: 10.1136/bjophthalmol-2011-300462.
[6] Van Cleynenbreugel H, Remeijer L, Hillenaar T. Descemet stripping automated endothelial keratoplasty: effect of intraoperative lenticule thickness on visual outcome and endothelial cell density[J]. Cornea, 2011, 30(11):1195-1200. DOI: 10.1097/ICO.0b013e31821821c7.
[7] Terry MA, Shamie N, Chen ES, et al. Precut tissue for Descemet’s stripping automated endothelial keratoplasty: vision, astigmatism, and endothelial survival[J]. Ophthalmology, 2009, 116(2):248-256. DOI: 10.1016/j.ophtha.2008. 09.017.
[8] Feizi S, Javadi MA. Effect of donor graft thickness on clinical outcomes after Descemet stripping automated endothelial keratoplasty[J]. J Ophthalmic Vis Res, 2019, 14(1):18-26. DOI: 10.4103/jovr.jovr_55_17.
[9] Romano V, Tey A, Hill NM, et al. Influence of graft size on graft survival following Descemet stripping automated endothelial keratoplasty[J]. Br J Ophthalmol, 2015, 99(6):784-788. DOI: 10.1136/bjophthalmol-2014-305648.
[10] Tang M, Stoeger C, Galloway J, et al. Evaluating DSAEK graft deturgescence in preservation medium after microkeratome cut with optical coherence tomography[J]. Cornea, 2013, 32(6):847-850. DOI: 10.1097/ICO. 0b013e31828 a27dd.
[11] Di Pascuale MA, Prasher P, Schlecte C, et al. Corneal deturgescence after Descemet stripping automated endothelial keratoplasty evaluated by Visante anterior segment optical coherence tomography[J]. Am J Ophthalmol, 2009, 148(1):32-37. DOI: 10.1016/j.ajo.2009.01.016.
[12] Lass JH, Benetz BA, Patel SV, et al. Donor, recipient, and operative factors associated with increased endothelial cell loss in the cornea preservation time study[J]. JAMA Ophthalmol, 2019, 137(2):185-193. DOI: 10.1001/ jamaophthalmol.2018.5669.
[13] Lekhanont K, Vanikieti K, Nimvorapun N, et al. Outcomes of descemet stripping automated endothelial keratoplasty using imported donor corneas[J/OL]. BMC Ophthalmol, 2017, 17(1):41[2022-04-15]. https://pubmed.ncbi.nlm.nih.gov/ 28381247/. DOI: 10.1186/s12886-017-0436-0.
[14] Busin M, Patel AK, Scorcia V, et al. Microkeratome-assisted preparation of ultrathin grafts for descemet stripping automated endothelial keratoplasty[J]. Invest Ophthalmol Vis Sci, 2012, 53(1):521-524. DOI: 10.1167/iovs.11-7753.
[15] Béal L, Navel V, Pereira B, et al. Efficacy of thin and ultrathin Descemet stripping automated endothelial keratoplasty and influence of graft thickness on postoperative outcomes: systematic review and meta-analysis[J]. Am J Ophthalmol, 2022, 240:170-186. DOI: 10.1016/j.ajo.2022.03.022.
[16] Durrani AF, Faith SC, Jhanji V. Ultrathin Descemet stripping automated endothelial keratoplasty[J]. Curr Opin Ophthalmol, 2019, 30(4):264-270. DOI: 10.1097/ICU.0000000000000575.
[17] Mencucci R, Favuzza E, Marziali E, et al. Ultrathin Descemet stripping automated endothelial keratoplasty versus Descemet membrane endothelial keratoplasty: a fellow-eye comparison[J/OL]. Eye Vis (Lond), 2020, 7:25[2022-04-15]. https://pubmed.ncbi.nlm.nih.gov/32391399/. DOI: 10.1186/ s40662-020-00191-6.