Abstract 【Download PDF】 【Read Full Text】
Objective
To investigate the mechanism of tissue damage caused by neutrophil matrix metalloproteinase-8 (MMP-8) in Fusarium keratitis.
Methods
A total of 108 male C57BL/6J SPF grade mice, 6-8 weeks old, were selected to establish a model of Fusarium keratitis (FK) in the right eyes.Corneal inflammation in mice was observed and scored under a slit lamp microscope.Based on the corneal inflammation scores, the modeling eyes were divided into 0, 12, 24, 48, and 72-hour groups post-modeling.At the corresponding time points, mice were euthanized, and corneal tissues were collected.The expressions of MMP-8, adenylate-activated protein kinase (AMPKα) and its serine 172-site phosphorylated form (p-AMPKα) proteins in corneal tissues were detected by Western blot.The neutrophil count in mice corneal tissues at each time point was determined using hematoxylin and eosin staining.The co-localization of neutrophils and MMP-8 protein in the cornea was observed by immunofluorescence staining.In the in vitro corneal collagen degradation experiment, corneal tissues were divided into MMP-8 group, buffer group, and normal saline group, which were treated with 100 μl of activated recombinant MMP-8, detection buffer, and normal saline, respectively.Hydroxyproline content in corneal tissues was determined using a hydroxyproline assay kit, and the mass fractions of hydroxyproline were compared among the groups.Peripheral blood neutrophils were isolated from human blood samples, and Fusarium spores were collected for experiments.Human neutrophils were divided into four groups, negative control group (cultured neutrophils), co-culture group (neutrophils co-cultured with spores), AICAR-treated group (neutrophils co-cultured with spores and treated with p-AMPK protein kinase activator AICAR), and compound C-treated group (neutrophils co-cultured with spores and treated with the inhibitor compound C).The MMP-8 protein expression levels in each group of human neutrophils were assessed via immunofluorescence staining.The use and care of animals complied with the ARVO statement and Regulations for the Administration of Affairs Concerning Experimental Animals.The animal experiment protocol was approved by the Animal Ethics Committee of Henan Eye Hospital (No.HNEECA-2017-04-02).One healthy adult volunteer was selected and 10 ml of peripheral venous blood was collected.The clinical study protocol was approved by the Clinical Ethics Committee of Henan Eye Hospital (No.HNEECKY-2019[16]).
Results
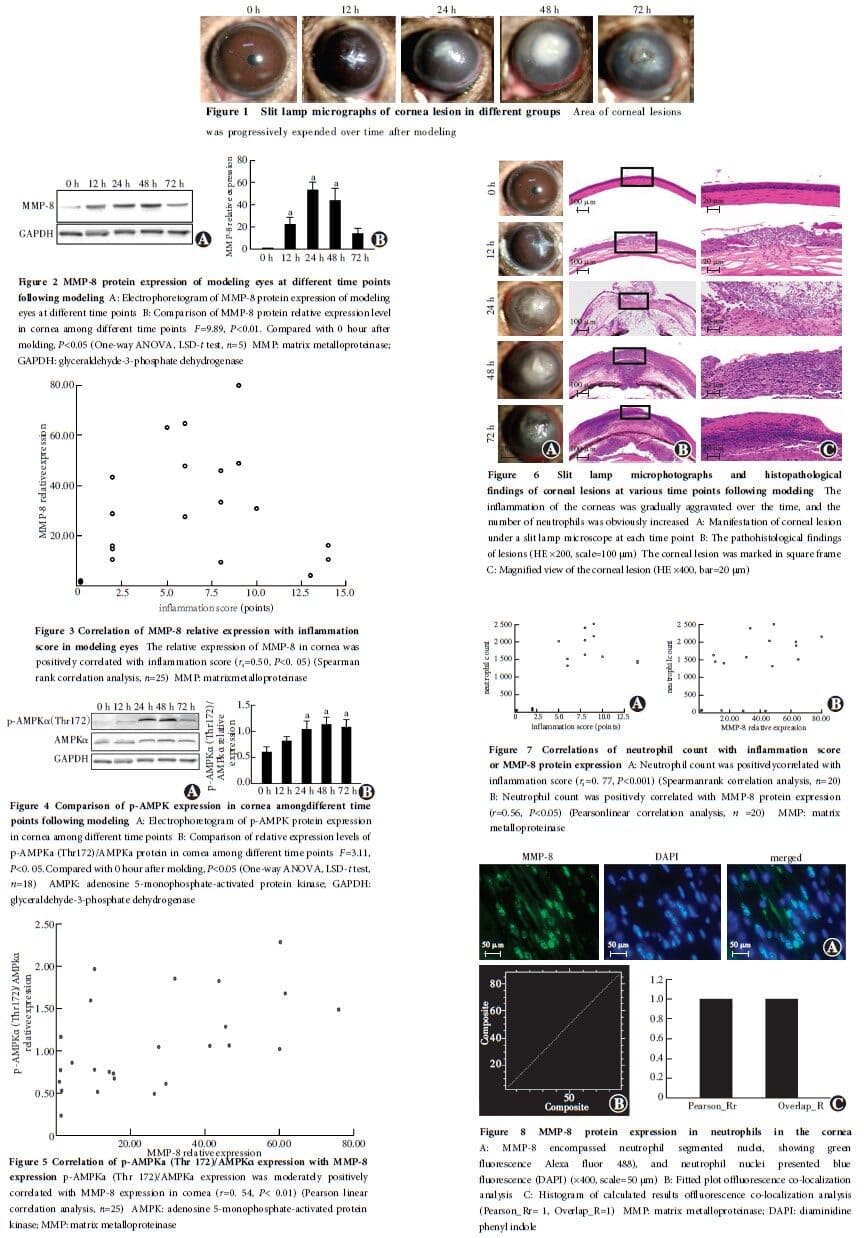
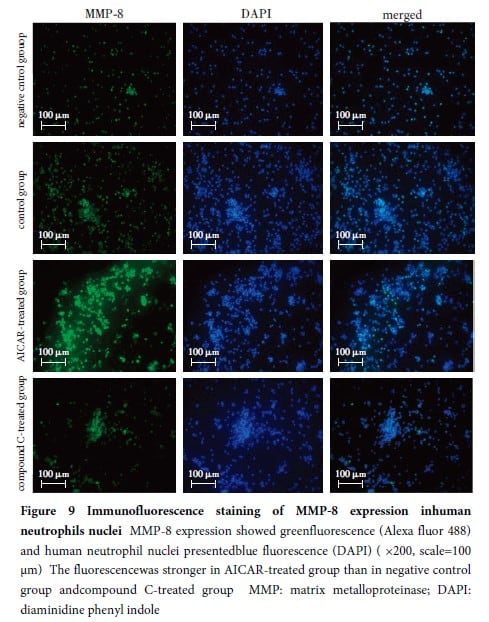
At 24 hours post-modeling, corneal opacification was observed in the modeling eyes, and corneal perforation occurred in 72-hour post-modeling group.The corneal inflammation scores in 24, 48, and 72-hour post-modeling groups were all higher than those in 12-hour post-modeling group, and the differences were statistically significant (all at P<0.001).The relative expression levels of MMP-8 protein in the cornea were higher in 12, 24, and 48-hour post-modeling groups compared to 0-hour group, with statistically significant differences (all at P<0.001).There was a moderate positive correlation between the relative expression level of MMP-8 protein in the cornea and the inflammation scores of the modeling eye (rs=0.50, P<0.05).In the cornea, the p-AMPKα (Thr 172)/AMPKα ratio was higher in 24, 48, and 72-hour post-modeling groups than in 0-hour group, and the differences were statistically significant (all at P<0.05).The p-AMPKα(Thr 172)/AMPKα ratio in the cornea was moderately positively correlated with the relative expression level of MMP-8 protein (r=0.54, P<0.01).The number of neutrophils in the cornea was significantly higher in 24, 48, and 72-hour post-modeling groups than in 0-hour group, with statistically significant differences (all at P<0.001).The number of neutrophils in the cornea was strongly positively correlated with the inflammation score (rs=0.77, P<0.001), and was moderately positively correlated with the relative expression level of MMP-8 protein (r=0.56, P<0.05).MMP-8 protein expression in the cornea of the modeling eyes showed a high degree of co-localization with neutrophils.The hydroxyproline content in the cornea was (0.52±0.02)μg/mg, (0.51±0.03)μg/mg, and (0.27±0.02)μg/mg in buffer group, normal saline group and MMP-8 group, respectively, with a significant overall difference among them (F=156.63, P<0.01).The corneal hydroxyproline content was lower in MMP-8 group compared to buffer and normal saline groups, and the differences were statistically significant (all at P<0.05).In the experiment involving the infection of cultured Fusarium spores with human neutrophils, the fluorescence intensity of MMP-8 expression was significantly higher in AICAR-treated group than in negative control group and compound C-treated group, with statistically significant differences (all at P<0.05).
Conclusions
The MMP-8 secreted by neutrophils in mice with fungal keratitis can degrade corneal stromal collagen fibers, leading to corneal opacification or perforation.The variations in MMP-8 protein expression levels in human neutrophils may be associated with AMPK activation.
Key words:
Figures&Tables
Contributor Information
Department of Ophthalmology, Henan Provincial People’s Hospital, Henan Eye Hospital, Henan Eye Institute, Henan University People’s Hospital, Zhengzhou University People’s Hospital, Zhengzhou 450003, China
Department of Ophthalmology, Henan Provincial People’s Hospital, Henan Eye Hospital, Henan Eye Institute, Henan University People’s Hospital, Zhengzhou University People’s Hospital, Zhengzhou 450003, China
Department of Ophthalmology, Henan Provincial People’s Hospital, Henan Eye Hospital, Henan Eye Institute, Henan University People’s Hospital, Zhengzhou University People’s Hospital, Zhengzhou 450003, China
Department of Ophthalmology, Henan Provincial People’s Hospital, Henan Eye Hospital, Henan Eye Institute, Henan University People’s Hospital, Zhengzhou University People’s Hospital, Zhengzhou 450003, China
Department of Ophthalmology, Henan Provincial People’s Hospital, Henan Eye Hospital, Henan Eye Institute, Henan University People’s Hospital, Zhengzhou University People’s Hospital, Zhengzhou 450003, China
Department of Ophthalmology, Henan Provincial People’s Hospital, Henan Eye Hospital, Henan Eye Institute, Henan University People’s Hospital, Zhengzhou University People’s Hospital, Zhengzhou 450003, China
Department of Ophthalmology, Henan Provincial People’s Hospital, Henan Eye Hospital, Henan Eye Institute, Henan University People’s Hospital, Zhengzhou University People’s Hospital, Zhengzhou 450003, China