·Clinical Research·
Use of a peripheral defocus soft contact lens to control myopia progression in children and adolescents: a meta-analysis
Liu Zhuzhu1, Wei Ruihua1, Zhang Xiangyu2, Pei Ruxia1
1Tianjin Key Laboratory of Retinal Functions and Diseases, Tianjin Branch of National Clinical Research Center for Ocular Disease, Eye Institute and School of Optometry, Tianjin Medical University Eye Hospital, Tianjin 300384, China; 2 Department of Traditional Chinese Medicine, Baokang Hospital, Tianjin University of TCM, Tianjin 300l93, China
Corresponding author: Wei Ruihua, Email: rwei@tmu.edu.cn
[Abstract] [View PDF in English] [View PDF in Chinese] [Read Full Text]
Objective This study aimed to evaluate the effect of peripheral defocus soft contact lenses (PDSCLs), single-vision spectacles, and single-vision contact lenses (SVCLs) on myopia progression in children and adolescents.
Methods A meta-analysis was conducted to collect relevant studies on the myopia control effect of PDSCLs in children. English databases including PubMed, Medline, Embase, and Cochrane Library were searched with myopia, contact lens, children, adolescents, myopia progression, axial length, and refractive error and relevant free English terms as keywords. Chinese databases, including China National Knowledge Infrastructure (CNKI), Wanfang database, and China Science and Technology Journal Database (VIP database), were searched with corresponding Chinese phrases and relevant free Chinese terms as keywords. Randomized controlled trials (RCTs) on myopia control effect in children and adolescents were selected, with PDSCL wearers as the experimental group and single-vision spectacles or SVCL wearers as the control group. The quality of included studies was evaluated using the Cochrane tool to assess the risk of bias for RCTs. The combined effects of the changes in refraction and axial length between experimental and control groups were determined using weighted mean difference (WMD) and 95% confidence interval (CI). The heterogeneity of included studies was evaluated using I2 statistics. The refraction and axial length in the experimental and control groups were compared using the Z test. The myopia control effect of the add powers of different PDSCLs was analyzed by the subgroup analysis. The experimental data with add power ≤ +2.00 D, low aberration, and low depth of focus were assigned to low-medium add power subgroup, and the experimental data with add power >+2.00, high aberration, and high depth of focus were assigned to high add power subgroup.
Results A total of 378 studies were retrieved. Finally, 10 high quality RCTs and 14 groups of data were included in this meta-analysis. In these studies, 1,645 myopic children aged 6-18 years were enrolled, including 808 in the experimental group and 837 in the control group. The follow-up ranged from 10 to 36 months. The 10 studies included two crossover trials without a washout period, so only the first intervention results were included. According to the meta-analysis, the change in refraction in the experimental group was significantly less than in the control group (WMD = 0.22D, 95% CI: 0.15–0.30, Z = 5.65; P < 0.05). The change in axial elongation was significantly less in the experimental group than in the control group (WMD = -0.10 mm, 95% CI: -0.12 to -0.09, Z = 12.28; P < 0.05). The subgroup analysis showed that the WMD of refraction change and axial elongation between the experimental and control groups were 0.21 D (95% CI: 0.10–0.31) and -0.10 mm (95% CI: -0.13 to 0.08) in the low-medium add power subgroup, respectively, and 0.26 D (95% CI: 0.13–0.38) and -0.13 mm (95% CI: -0.15 to 0.10) in the high add power subgroup, respectively.
Conclusions PDSCLs have a better myopia control effect than single-vision spectacles or SVCLs in children and adolescents. When the add power was higher, PDSCLs slowed the progression of myopia more effectively.
[Keywords] Adolescent; axial length; child; contact lenses; meta-analysis; myopia; myopic defocus; peripheral defocus; prevention and control; refraction
Fund program: Social Science Major Project of Tianjin Municipal Education Commission (2020JWZD20)
DOI: 10.3760/cma.j.cnll5989-20210521-00314
In recent years, myopia has become a global public health problem. The global prevalence of myopia is predicted to reach 50% in 2050[1]. Myopia is an irreversible eye disease, which can easily lead to retinal detachment, macular disease, macular hemorrhage, choroidal neovascularization, and other ocular complications after it progresses to high myopia, and is one of the important reasons for visual impairment and even blindness[2-3]. How to prevent and control myopia in children and adolescents has become an important societal issue. At present, the mechanism of myopia has not been fully clarified, but the visual feedback mechanism plays an essential role in the development of ocular refraction. Previous studies believed that the optical signal of the fovea of the retina controlled the growth of the eyeball. However, subsequent animal experiments proved that the peripheral retinal signal played a major role in regulating the growth of the eyeball, that is, the eyeball developed into axial myopia under the guidance of the peripheral hyperopia defocus signal, and remained hyperopia under the guidance of the myopic defocus signal[4-5]. This theory provided a basis for the application of peripheral defocus soft contact lenses (PDSCLs) and other optical means in myopia control. PDSCLs were initially used to correct presbyopia [6]. In recent years, PDSCLs designed with new materials and technologies, such as concentric ring bifocal or progressive multifocal, new spherical aberration, extended focal depth design, and PDSCLs with different add power, have been proven to effectively delay myopia progression in children and adolescents [7-15]. Many recent studies have investigated the effect of PDSCLs on myopia progression in children and adolescents. However, the results of these studies vary significantly, and the quality of the studies is mixed. Hence, they do not provide reasonable guidance to ophthalmologists. This study aimed to conduct a meta-analysis of the randomized controlled trials (RCTs) of PDSCLs and single-vision contact lenses (SVCLs), so as to improve the understanding of the myopia control effect of PDSCLs in children and adolescents.
1 Materials and Methods
1.1 Search strategy
Two researchers searched PubMed, Medline, Embase, Cochrane Library, CNKI, Wanfang Data, and Weibo to collect relevant data on the effect of PDSCLs in myopia control in children and adolescents. The search was conducted from the establishment of the database until April 2021, and the studies retrieved were in Chinese and English. A combination of subject headings and free text terms was used in the search, including myopia, contact lens, children, adolescents, myopia progression, axial length, refractive error, and related free terms. The researchers manually retrieved and traced the references included in the studies to supplement and obtain additional relevant studies. The ongoing clinical trials were registered by China Clinical Trial Registration Center, the World Health Organization International Clinical Trial Registration Platform, and ClinicalTrials.gov, and were also searched.
1.2 Selection of studies
The inclusion criteria were as follows: (1) RCTs with either a parallel control or crossover design; (2) subjects: children and adolescents aged 6–18 years with myopia; (3) PDSCLs with the unique design used in the experimental group, and single-vision spectacles or SVCLs used in the control group; and (4) all studies including the outcome data of the first stage (10 or 12 months), because several studies reported that PDSCLs had a time effect on myopia control during the follow-up period [14]. The exclusion criteria were as follows: (1) evaluation of other interventions such as rigid breathable contact lenses and refractive surgery; (2) non-RCT studies; and (3) incomplete data or repeated research.
1.3 Data extraction and quality assessment
Two researchers independently screened studies and conducted data extraction, methodological quality evaluation, and cross-checking. The extracted information included author, publication year, country or region, PDSCL design type, follow-up time, age, baseline refraction, sample size, loss to follow-up, intervention methods in the control group, and outcome indicators. The primary outcome measure was the change in equivalent spherical equivalent (SER) at different visits, and the secondary outcome measure was the change in axial length (AL) at different visits. The experimental data with add power ≤+2.00 D, low aberration, and low depth of focus were assigned to the low-medium add power subgroup, and the experimental data with add power >+2.00, high aberration, and high depth of focus were assigned to the high add power subgroup to compare the myopia control effect of different PDSCLs add powers. We used GetDataGraph Digitizer 2.24 software (http: //Getdata graph digitizer. com) to read data from different follow-up periods.
The quality assessment of all included studies was independently performed by two researchers using the improved Cochrane risk-of-bias evaluation tool, which included the generation of random sequences, hiding of allocation schemes, blinding of researchers and subjects, blinding of outcome evaluators, dropout and dropout reports, selective reporting of research results, and other sources of bias. The judgment level was categorized into high bias risk, low bias risk, and uncertainty. Any disagreement between the two researchers on the aforementioned assessment was resolved through discussion or consultation with the third researcher.
1.4 Statistical analysis
Review ManagerVersion 5.3 (http: //Tech.cochrane.org/revman) was used for data analysis. The differences between the two groups in terms of the changes in SER and AL were expressed using weighted mean difference (WMD) and 95% confidence interval (CI). The myopia control rate was defined as the ratio of the difference in refraction change (axial elongation) between the two groups and refraction change (axial elongation) in the control group. The I2 test was used to evaluate the statistical heterogeneity in the study. A P value <0.1 or I2 >50% was significant for the heterogeneity test. A random-effects model (I-V heterogeneity) was used to analyze the outcome indicators. A P value >0.1 or I2 <50% indicated no significant difference in the heterogeneity test between studies. A fixed-effect model (Mantel–Haenszel) was used to evaluate the combined effect. The double-tailed test was used, and a P value <0.05 indicated a statistically significant difference.
2 Results
2.1 Selection of studies
We retrieved 378 studies, excluding 78 repetitive studies and 290 studies that did not meet the inclusion criteria. Finally, 10 RCTs were included. The process of study retrieval and screening is shown in Figure 1.
2.2 Study characteristics
In the 10 RCTs included, the participants were aged 6−18 years. The study areas covered various countries and regions, including the United States, Chinese Mainland, Hong Kong, New Zealand, Japan, Spain, Portugal, Canada, Singapore, and the United Kingdom. Among these studies, the studies by Anstice et al.[16] and Fujikado et al.[17] were crossover trials. Considering no washout period between the two visits, only the results of the first period were included for analysis. The study of Walline et al.[7] included the data of two groups with add power +2.50 and +1.50 D. Sankaridurg et al.[18] included four groups of progressive design with add power of +2.50 and +1.50 D and extended focal depth of +1.75 and +1.25 D, respectively. Therefore, 14 groups of data were included in this study. The design types of PDSCLs included concentric bifocal, progressive multifocal, new extended focal depth, and positive spherical aberration PDSCLs; 808 myopic children were included in the experimental group and 837 myopic children in the control group. The dropout rates in the experimental and control groups ranged from 0% to 43% (Table 1).

2.3 Trial quality
Cheng et al.[8] only mentioned random trials and did not specifically describe the methods of random allocation and hiding. Fujikado et al.[17] only mentioned the use of a random number table, but did not specifically describe the method of randomized hiding. The study by Anstice et al.[16] was a crossover and single-blind trial due to the characteristic of the design itself. Because Ruiz Pomeda et al.[19] used the single-vision spectacles in the control group, the trial adopted single blinding. Each study specifically described the number and reasons for dropouts during the follow-up. The quality assessments of these studies are shown in Figures 2 and 3.


2.4 Refraction, AL changes, and control rate in the experimental and control groups
The myopia control rate of PDSCLs with different designs and different add powers ranged from 12% to 72%, and the axial elongation control rate ranged from 12% to 79%. Among these, the participants in the study by Aller et al.[15] included only children with esophoria myopia, resulting in a significantly higher myopia control rate of 72% and axial growth control rate of 79% compared with those in other studies. Walline et al.[7] reported lower myopia control rate (15%) and axial growth control rate (12%) in the low add power group (+1.50 D) compared with other studies (Table 2).

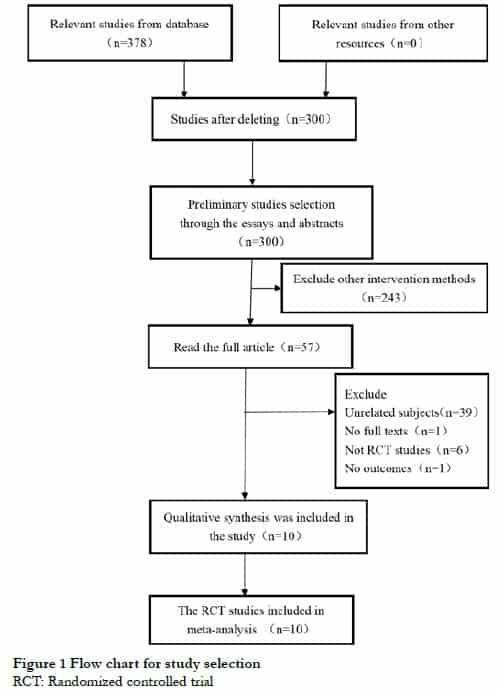
2.5 Analysis of refraction change
A random-effects model was used due to the obvious heterogeneity of refraction changes among the 14 groups of data (I2 = 87%, P < 0.05). The WMD of refraction change between the two groups was 0.22 D (95% CI: 0.15-0.30), with a statistically significant difference (Z = 5.65, P < 0.05) (Figure 4). However, after the exclusion of the studies by Chamberlain et al.[14] and Aller et al.[15], which included both eyes of patients and participants with esophoria myopia, respectively, the heterogeneity was significantly reduced (I2 = 0%, P > 0.05). The WMD of refraction change in the two groups was 0.18 D (95% CI: 0.14–0.22); the difference was still statistically significant between the two groups (Z = 9.18, P < 0.05) (Figure 5).
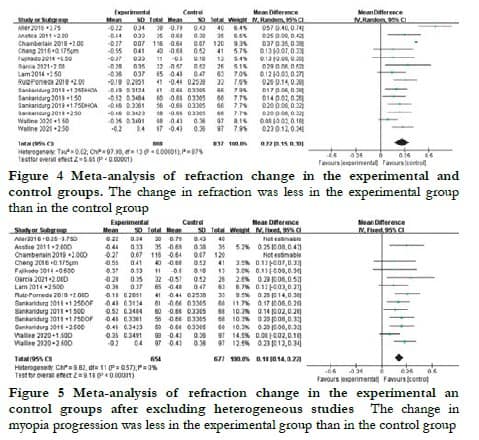
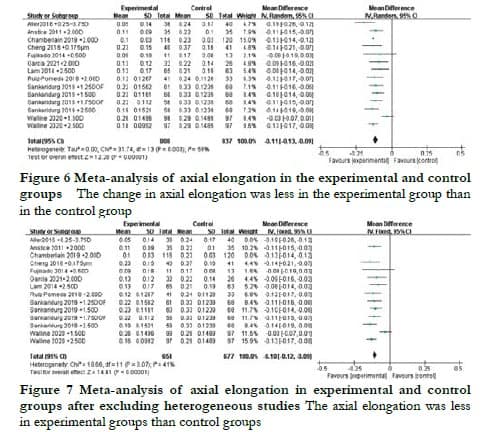
2.6 Analysis of axial elongation
The change in AL among the 14 groups of data analyzed showed significant heterogeneity (I2 = 59%, P < 0.05). Thus, a random-effects model was used to evaluate the WMD of AL change between the experimental and control groups, which was found to be -0.11 mm (95% CI: -0.13 to 0.09) with a statistically significant difference (Z = 12.28, P < 0.05) (Figure 6). However, after excluding the studies by Chamberlain et al. [14] and Aller et al.[15], the heterogeneity decreased significantly (I2 = 41%, P = 0.07). A fixed-effects model was used to evaluate the WMD of AL change, which was found to be –0.10 mm (95% CI: -0.12 to -0.09) with a statistically significant difference (Z = 12.28, P < 0.05) (Figure 7).
2.7 Treatment effect of PDSCLs with different add power subgroups
In the low-medium add power subgroups, the WMD of refraction change and AL change between the two groups was 0.21 D (95% CI: 0.10–0.31) and -0.10 mm (95% CI: -0.13 to -0.08), respectively. In the high add power subgroup, the WMD of refraction and AL change between the two groups was 0.26 D (95% CI: 0.13–0.38) and -0.13 mm (95% CI: -0.15 to -0.10), respectively (Figures 8 and 9).

3 Discussion
Evidence-based medicine research showed that RCT design could provide stronger research evidence for clinical research. The studies included in this analysis incorporated various types of PDSCLs. The concentric ring bifocal PDSCLs were composed of a farsighted central area surrounded by a series of concentric ring structures consisting of defocused areas and correction areas. The progressive PDSCLs featured a central distance zone with a gradual transition refractive power, ultimately reaching a progressive positive power in peripheral areas. Additionally, the study introduced new PDSCLs designed with +0.175-µm positive spherical aberration at 5 mm around the optical center, specifically to counteract the negative spherical aberration during accommodation[8]. Finally, the PDSCLs with extended focal depth were designed based on selective higher-order aberrations, and a non-single and aperiodic refractive distribution was used to expand the focal depth and optimize retinal quality[18]. This study conducted a meta-analysis on 10 RCTs, including 14 groups of PDSCL experimental data with different designs on myopia progression in children and adolescents aged 6-18 years. The findings of this study suggested that PDSCLs could effectively control myopia in children and adolescents by slowing myopia progression by 0.22 D per year and axial elongation by 0.11 mm per year, compared with those in the control group. After excluding two studies with obvious heterogeneity, the heterogeneity was significantly reduced and the PDSCLs were found to slow myopia progression by 0.18 D and axial elongation by 0.10 mm every year compared with those in the control group. These findings supported the results obtained from animal models, suggesting that peripheral myopic defocusing around the retina could slow the progression of myopia and axial elongation[20]. However, other studies showed that retinal relative peripheral hyperopic defocusing could not predict the occurrence or progression of myopia in children[21]. A recent study investigated the impact of the monocular correction on children with progressive myopia. According to the findings, the uncorrected eye experiences prolonged myopic defocusing throughout the entire retina, compared with the fully corrected contralateral eye. This phenomenon could be responsible for slower myopia progression and eye axis growth when myopia was left uncorrected, as it created a myopic defocusing state in the retina for both near and far vision. Notably, this state differed from the myopic defocusing state that occurred when only far vision was affected in both eyes.[22]. Therefore, larger-scale and longer-term studies are needed to explore the changes in peripheral retinal refraction and the effects of PDSCLs on myopia in children and adolescents.
The use of PDSCLs was shown to alter the peripheral refractive state of the retina. Allinjawi et al.[23] demonstrated that different add powers (+1.50, +2.50, +3.00, and +3.50 D, temporal 35° to nasal 35°) of multifocal contact lenses reduced peripheral hyperopic defocus with increasing add power. Besides peripheral retinal myopic defocusing, spherical aberration and extended depth of focus were also considered important optical factors for slowing myopia progression [24-26]. These findings suggested that the asymmetry of retinal optical signals might play an important role in slowing the progression of myopia and axial elongation. This study also investigated new PDSCLs designed with positive spherical aberration and extended focal depth, which were classified into low-medium add power and high add power subgroups. The subgroup analysis revealed that higher add power PDSCLs demonstrated better myopia control effects. The BLINK study also demonstrated that higher add power (+2.50 D) had a more significant advantage in slowing down myopia progression compared with medium add power (+1.50 D) and SVCLs[7].
The myopia control rates of PDSCLs in the present study varied from 12% to 72% across different follow-up durations. However, long-term follow-up studies on the effects of PDSCLs on myopia control were lacking. A 3-year randomized, double-blind clinical trial by Chamberlain et al.[14] showed that PDSCLs had the most prominent effect on myopia control in the first year of the study, but the effect continued to accumulate throughout the entire observation period (12, 24, and 36 months). Similarly, other 2-year follow-up studies also showed a sustained effect of PDSCLs on myopia control[10, 13, 27]. These findings were inconsistent with the results of the COMET study, which demonstrated that progressive spectacles could only slow myopia progression during the first year of follow-up[28]. Although the treatment effect of PDSCLs might last for 36 months or more, it appeared to peak during 12–24 months of intervention, which was consistent with similar studies on multifocal spectacle lenses or rigid corneal contact lenses[28-30]. This might be related to the counteraction of ocular changes, such as corneal flattening caused by contact lenses, slow progression of myopia with the aging of children, or decreased patient compliance during treatment.
Several research findings suggested that the impact of PDSCLs on myopia control was more noticeable among individuals who wore them for a longer time or exhibited high compliance[13]. Since the myopic defocusing in the peripheral retina of PDSCL wearers is influenced by the diameter of the optical zone and pupil size, personalized treatment with specific additional power and optical diameters of PDSCLs based on individual pupil diameter and accommodation lag may be a more effective approach for myopia control. Although this analysis included high-quality RCTs, potential biases could not be completely eliminated. Additionally, some studies suggested that myopia progression was associated with factors such as race, sex, age, baseline myopia, and design features of the lenses, including aspherical surfaces, foveal defocus for near or distant objects, and magnitude of near addition[29, 31, 32]. However, other studies suggested that the myopia control effect of PDSCLs was universal and not influenced by these factors[14]. A majority of studies on PDSCLs included in this review were conducted on White children with low myopia. However, PDSCLs appeared to have a more significant effect in controlling myopia in Asian children who typically spent less time outdoors. Therefore, the subgroup analyses on these factors were not feasible in this study.
To sum up, PDSCLs have a better myopia control effect than single-vision spectacles or SVCLs in children and adolescents, and PDSCLs with higher add power can slow the progression of myopia more effectively.
Conflict of interests All authors declare no conflicts of interest.
Acknowledgments Liu Zhuzhu: Collected data, participated in topic selection, collated and analyzed data, and wrote the manuscript; Zhang Xiangyu: participated in data collation; Pei Ruxia: did paper revision; Wei Ruihua: participated in topic selection, thesis revision, and finalization.
References
[1] Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050[J]. Ophthalmology, 2016, 123(5):1036-1042. DOI: 10.1016/j.ophtha.2016.01.006.
[2] Sankaridurg P, Conrad F, Tran H, et al. Controlling progression of myopia: optical and pharmaceutical strategies[J]. Asia Pac J Ophthalmol (Phila), 2018, 7(6):405-414. DOI: 10.22608/APO.2018333.
[3] Ohno-Matsui K, Lai TY, Lai CC, et al. Updates of pathologic myopia[J]. Prog Retin Eye Res, 2016, 52:156-187. DOI: 10.1016/j.preteyeres.2015.12.001.
[4] Smith EL 3rd, Hung LF, Huang J. Relative peripheral hyperopic defocus alters central refractive development in infant monkeys[J]. Vision Res, 2009, 49(19):2386-2392. DOI: 10.1016/j.visres.2009.07.011.
[5] Liu Y, Wildsoet C. The effect of two-zone concentric bifocal spectacle lenses on refractive error development and eye growth in young chicks[J]. Invest Ophthalmol Vis Sci, 2011, 52(2):1078-1086. DOI: 10.1167/iovs.10-5716.
[6] Charman WN. Developments in the correction of presbyopia I: spectacle and contact lenses[J]. Ophthalmic Physiol Opt, 2014, 34(1):8-29. DOI: 10.1111/opo.12091.
[7] Walline JJ, Walker MK, Mutti DO, et al. Effect of high add power, medium add power, or single-vision contact lenses on myopia progression in children: The BLINK randomized clinical trial[J]. JAMA, 2020, 324(6):571-580. DOI: 10.1001/jama.2020.10834.
[8] Cheng X, Xu J, Chehab K, et al. Soft contact lenses with positive spherical aberration for myopia control[J]. Optom Vis Sci, 2016, 93(4):353-366. DOI: 10.1097/OPX.0000000000000773.
[9] Pauné J, Queiros A, Quevedo L, et al. Peripheral myopization and visual performance with experimental rigid gas permeable and soft contact lens design[J]. Cont Lens Anterior Eye, 2014, 37(6):455-460. DOI: 10.1016/j.clae.2014.08.001.
[10] Pauné J, Morales H, Armengol J, et al. Myopia control with a novel peripheral gradient soft lens and orthokeratology: A 2-year clinical trial[J/OL]. Biomed Res Int, 2015, 2015:507572[2021-11-28]. http://www.ncbi.nlm.nih.gov/pubmed/26605331. DOI: 10.1155/2015/507572.
[11] Sankaridurg P, Holden B, Smith E 3rd, et al. Decrease in rate of myopia progression with a contact lens designed to reduce relative peripheral hyperopia: one-year results[J]. Invest Ophthalmol Vis Sci, 2011, 52(13):9362-9367. DOI: 10.1167/iovs.11-7260.
[12] Garcia-Del Valle AM, Blázquez V, Gros-Otero J, et al. Efficacy and safety of a soft contact lens to control myopia progression[J]. Clin Exp Optom, 2021, 104(1):14-21. DOI: 10.1111/cxo.13077.
[13] Lam CS, Tang WC, Tse DY, et al. Defocus incorporated soft contact (DISC) lens slows myopia progression in Hong Kong Chinese schoolchildren: A 2-year randomised clinical trial[J]. Br J Ophthalmol, 2014, 98(1):40-45. DOI: 10.1136/bjophthalmol-2013-303914.
[14] Chamberlain P, Peixoto-de-Matos SC, Logan NS, et al. A 3-year randomized clinical trial of MiSight lenses for myopia control[J]. Optom Vis Sci, 2019, 96(8):556-567. DOI: 10.1097/OPX.0000000000001410.
[15] Aller TA, Liu M, Wildsoet CF. Myopia control with bifocal contact lenses: a randomized clinical trial[J]. Optom Vis Sci, 2016, 93(4):344-352. DOI: 10.1097/OPX.0000000000000808.
[16] Anstice NS, Phillips JR. Effect of dual-focus soft contact lens wear on axial myopia progression in children[J]. Ophthalmology, 2011, 118(6):1152-1161. DOI: 10.1016/j.ophtha.2010.10.035.
[17] Fujikado T, Ninomiya S, Kobayashi T, et al. Effect of low-addition soft contact lenses with decentered optical design on myopia progression in children: a pilot study[J]. Clin Ophthalmol, 2014, 8:1947-1956. DOI: 10.2147/OPTH.S66884.
[18] Sankaridurg P, Bakaraju RC, Naduvilath T, et al. Myopia control with novel central and peripheral plus contact lenses and extended depth of focus contact lenses: 2 year results from a randomised clinical trial[J]. Ophthalmic Physiol Opt, 2019, 39(4):294-307. DOI: 10.1111/opo.12621.
[19] Ruiz-Pomeda A, Pérez-Sánchez B, Valls I, et al. MiSight assessment study Spain (MASS). A 2-year randomized clinical trial[J]. Graefes Arch Clin Exp Ophthalmol, 2018, 256(5):1011-1021. DOI: 10.1007/s00417-018-3906-z.
[20] Smith EL 3rd, Kee CS, Ramamirtham R, et al. Peripheral vision can influence eye growth and refractive development in infant monkeys[J]. Invest Ophthalmol Vis Sci, 2005, 46(11):3965-3972. DOI: 10.1167/iovs.05-0445.
[21] Mutti DO, Sinnott LT, Mitchell GL, et al. Relative peripheral refractive error and the risk of onset and progression of myopia in children[J]. Invest Ophthalmol Vis Sci, 2011, 52(1):199-205. DOI: 10.1167/iovs.09-4826.
[22] Phillips JR. Monovision slows juvenile myopia progression unilaterally[J]. Br J Ophthalmol, 2005, 89(9):1196-1200. DOI: 10.1136/bjo.2004.064212.
[23] Allinjawi K, Kaur S, Akhir SM, et al. Inverting peripheral hyperopic defocus into myopic defocus among myopic schoolchildren using addition power of multifocal contact lens[J]. Saudi J Ophthalmol, 2020, 34(2):94-100. DOI: 10.4103/1319-4534.305035.
[24] Kang P, Swarbrick H. Peripheral refraction in myopic children wearing orthokeratology and gas-permeable lenses[J]. Optom Vis Sci, 2011, 88(4):476-482. DOI: 10.1097/OPX.0b013e31820f16fb.
[25] Hiraoka T, Kakita T, Okamoto F, et al. Influence of ocular wavefront aberrations on axial length elongation in myopic children treated with overnight orthokeratology[J]. Ophthalmology, 2015, 122(1):93-100. DOI: 10.1016/j.ophtha.2014.07.042.
[26] Berntsen DA, Barr JT, Mitchell GL. The effect of overnight contact lens corneal reshaping on higher-order aberrations and best-corrected visual acuity[J]. Optom Vis Sci, 2005, 82(6):490-497. DOI: 10.1097/01.opx.0000168586.36165.bb.
[27] Walline JJ, Greiner KL, McVey ME, et al. Multifocal contact lens myopia control[J]. Optom Vis Sci, 2013, 90(11):1207-1214. DOI: 10.1097/OPX.0000000000000036.
[28] Gwiazda J, Hyman L, Hussein M, et al. A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children[J]. Invest Ophthalmol Vis Sci, 2003, 44(4):1492-1500. DOI: 10.1167/iovs.02-0816.
[29] Li SM, Ji YZ, Wu SS, et al. Multifocal versus single vision lenses intervention to slow progression of myopia in school-age children: A meta-analysis[J]. Surv Ophthalmol, 2011, 56(5):451-460. DOI: 10.1016/j.survophthal.2011.06.002.
[30] Walline JJ, Jones LA, Mutti DO, et al. A randomized trial of the effects of rigid contact lenses on myopia progression[J]. Arch Ophthalmol, 2004, 122(12):1760-1766. DOI: 10.1001/archopht.122.12.1760.
[31] Hyman L, Gwiazda J, Hussein M, et al. Relationship of age, sex, and ethnicity with myopia progression and axial elongation in the correction of myopia evaluation trial[J]. Arch Ophthalmol, 2005, 123(7):977-987. DOI: 10.1001/archopht.123.7.977.
[32] Li SM, Kang MT, Wu SS, et al. Efficacy, safety and acceptability of orthokeratology on slowing axial elongation in myopic children by meta-analysis[J]. Curr Eye Res, 2016, 41(5):600-608. DOI: 10.3109/02713683.2015.1050743.