·Clinical Research·
Distribution characteristics of choroidal thickness in normal population and the diagnostic cut–off value study for pachychoroid
Zhang Xinyuan1&, Qiu Bingjie1, Wang Yanhong2, Li Zhiqing3, Zeng Yiyun1, Chen Xiaosi1
1Beijing Tongren Eye Center, Beijing Tongren Hospital, Capital Medical University, Beijing Key Laboratory of Institute of Ophthalmology and Visual Sciences, Beijing 100730, China; 2Department of Epidemiology and Biostatistics, Institute of Basic Medical Sciences Chinese Academy of Medical Sciences & School of Basic Medicine Peking Union Medical College, Beijing 100005, China; 3Tianjin Key Laboratory of Retinal Function sand Diseases, Tianjin Branch of National Clinical Research Center for Ocular Disease, Eye Institute and School of Optometry, Tianjin Medical University Eye Hospital, Tianjin 300384, China
&Corresponding author: Zhang Xinyuan, Email: mmzxy2010@163.com
[Abstract] [View PDF in English] [View PDF in Chinese] [Read Full Text]
Objective To characterize the distribution characteristics of choroidal thickness in healthy normal subjects and to define the diagnostic cut-off value for pachychoroid.
Methods A cross-sectional study was performed. 446 eyes of 230 healthy subjects from the pachychoroid spectrum disorder (PCD) cohort in Beijing Tongren Hospital were enrolled for the choroidal thickness distribution analysisfrom April 2018 to June 2021. 314 eyes of 274 patients with PCD including 149 eyes of 113 patients with central serous chorioretinopathy (CSC), 95 eyes of 81 patients with polypoid choroidal vasculopathy (PCV), 70 eyes of 60 patients with neovascular age-related macular degeneration (nAMD), along with 382 eyes of 199 normal subjects matched diopter, age and gender with PCD were selected for likelihood ratio analysis. General eye examinations including the best corrected visual acuity (BCVA), intraocular pressure, slit-lamp microscopy, dilated fundus examination and color fundus photography were carried out in all subjects. Swept-source optical coherence tomography (SS-OCT) of 9 mm×9 mm scanning mode was used to measure the subfoveal choroidal thickness (SFCT) automatically in nine macular regions according to the Early Treatment Diabetic Retinopathy Study classification system using TOPCON Advanced Boundary Segmentation (TABS) software. Pearson linear correlation analysis or Spearman rank correlation analysis were adopted to estimate the relationship of SFCT with age, gender, diopter. Multiple linear regression model was applied to analyze the variables affecting SFCT. After adjust age and refractive diopter, likelihood ratio test was used to determine the diagnostic cut-off value for pachychoroid.
Results A negative correlation was found between SFCT and age in normal eyes (r=-0.34, P<0.001), in both normal male and female groups (r=-0.43, P<0.001; r=-0.38; P<0.001). A weakly positive correlation was found between SFCT and diopter (rs=0.19, P<0.001). Gender, age and diopter were strongly correlated with SFCT (both at P<0.001). The diagnostic value range of pachychoroid in 20-39 years group, 40-59 years group, 60-79 years group and ≥80 years group was 320-330 μm, 330-340 μm, 250-275 μm and 200-225 μm, respectively. The percentage of eyes with pachychoroid in 20-39 years group, 40-59 years group and ≥60 years group was 14.71% (10/68), 24.48% (47/192) and 28.89% (55/184), respectively, showing statistically significant differences between different age groups (c2=6.170, P=0.046; LR=6.579, P=0.037). The proportion of pachychoroid in ≥60 years group was significantly higher than that of 20-39 years group, showing a statistically significant difference (χ2=5.982, P=0.014; LR=6.497, P=0.011).
Conclusions The distribution characteristics of pachychoroid vary in normal subjects over age. Age and diopter are the independent influencing factors of SFCT.
[Key words] Choroid diseases/diagnosis; Choroidal thickness; pachychoroidal; Tomography, optical coherence; Normal subject; Chinese; Likelihood ratio test; swept-source optical coherence tomography
Fund program: National Natural Science Foundation of China (81170859, 81570850, 81561128015, 82070988); The Ministry of Science and Technology Foundation of China (2016YFC1305600)
DOI: 10.3760/cma.j.cn115989-20220401-00127
Pachychoroid spectrum disorder (PCD) is a group of disorders characterized by chronic choroid thickening and dysfunction of choroidal vasculature. PCD is a relative new concept proposed by the tremendous progress of optical coherent tomography (OCT) technology[1-2]. In 2017, we introduced the concept of PCD into China for the first time and standardized its Chinese naming[2]. As the main source of nutrition for photoreceptors, structural and functional changes in the choroid play an important role in the pathogenesis of various retinal and choroidal diseases. In recent years, with the continuous advancement of fundus imaging technology, the measurement and quantification of choroidal biological parameters and their relationship with eye diseases have drawn widespread attention and contribute to a deeper understanding of the pathogenesis of various retinal and choroidal diseases. Among the variable choroidal parameters, choroidal thickness can more intuitively show the morphological changes of the choroid and can be used as an important imaging marker for the diagnosis of retinal and choroidal diseases, evaluation of intervention effects and follow-up monitoring. Since 1979, various examination methods/devices have been used for the measurement of choroidal thickness, such as ultrasonography, magnetic resonance imaging (MRI), and optical coherent tomography (OCT). Compared with ultrasonography and MRI, OCT measurement, especially swept source OCT (SS-OCT), is not only convenient and fast, but also has incomparable advantages in the accuracy of quantitative measurement[3-4]. With the improvement of the resolution of OCT scanning, the imaging of choroidal structures, especially the choroid-scleral boundary and choroidal vasculature can be described more clearly. At present, measurement of choroidal thickness mainly includes manual and automatic segmentation method by using different performance of OCT equipment[5], and various methods such as mean±standard deviation are also used to determine the cut-off value of pachychoroid in previous studies[6-7]. Since there is no uniform standard for the quantitative diagnostic criteria for pachychoroid, the diagnosis of PCD and treatment evaluation of some diseases are uncertain, and it is difficult to evaluate and compare clinical research results among the similar studies. In addition, related studies did not consider the confounding factors which may influence the choroidal thickness such as refractive status, age and gender, and the sensitivity, accuracy, specificity, and reproducibility of choroidal thickness measurement were not taken into account, conclusions from these studies are limited. PCD is still a newly recognized spectrum disease, precise measurement of choroidal thickness and the definition of the diagnostic value of pachychoroid are undoubtedly of great significance to the understanding of its pathogenesis and the diagnosis and prevention of related diseases. Currently, there still lack methods for standardized acquisition and accurate quantification of choroidal thickness in this research field. This study aims to characterize the distribution characteristics of choroidal thickness in healthy normal subjects and to define the diagnostic cut-off value for pachychoroid in normal people in a limited population in my country.
1 Materials and methods
1.1 General information
This was a cross-sectional study design. The distribution characteristics of choroidal thickness in 230 normal subjects and 446 eyes in the PCD cohort was recruited in Beijing Tongren Hospital from April 2018 to June 2021, including 95 males (186 eyes), 135 females (160 eyes), aged 22-88 years, with an average age of (55.52±15.07) years. 199 subjects (382 eyes) of whom were matched with the PCD group in diopter, age and gender were selected as the normal group to evaluate the diagnostic cut off value of pachychoroid including 116 males (221 eyes) and 83 females (161 eyes) , age ranged from 25 to 88 years, with an average of (56.57±15.53) years old. 314 eyes of 274 patients with PCD were selected as PCD group, including 176 males (204 eyes) and 98 females (110 eyes), aged 24-94 years, with an average of (58.68±14.96) years old. The PCD group consisted of 149 eyes of 113 subjects with central serous chorioretinopathy (CSC), 95 eyes of 81 cases of polypoidal choroidal vasculopathy (PCV), and 70 eyes of 60 subjects with neovascular age-related macular degeneration (nAMD). CSC group included 88 males (102 eyes), and 45 females (47 eyes), aged 24-73 years, with an average of (47.27±10.06) years; 53 males (62 eyes), and 28 females (33 eyes), aged 49-94 years. The average age was (67.13±10.55) years were enrolled in this PCV group; among the nAMD patients, there were 35 males with 40 eyes and 25 females with 30 eyes.
The inclusion criteria of healthy eyes for likelihood ratio test were: (1) age and sex matched with PCD group; (2) no other eye diseases by intensive eye examination. Exclusion criteria for healthy eyes: (1) those with any eye disease; (2) those with serious systemic diseases can’t tolerate eye examinations. Inclusion criteria for PCD patients were: (1) Patients diagnosed with PCV, nAMD or CSC by ophthalmic clinical examination. The diagnostic criteria for PCV based on the expert consensus of the PCV Working Group of the Asia-Pacific Ophthalmological Imaging Society in 2020[8], EVEREST study II and modified EVEREST criteria; CSC was diagnosis based on the typical clinical manifestations, fluorescein fundus angiography and indocyanine angiography, and typical imaging features of OCT[9]; the diagnosis of nAMD was based on the “Expert Consensus” by Spaide et al. The exclusion criteria for PCD were: (1) Diagnosed with other eye diseases that affect choroidal thickness, especially retinal choroidal vascular diseases, such as uveitis, choroidal neovascularization induced by pathological myopia, choroidal hemangioma, diabetic retinopathy, etc.; (2) patients with open-angle glaucoma or angle-closure glaucoma; (3) patients who have received anti-vascular endothelial growth factor therapy in the past six months; (4) patients who have received laser retinal photocoagulation or photodynamic therapy in the macular area; (5) patients have undergone surgery on the posterior segment of other eyes; (6) those who have opacity of the refractive media to obtain a clear image; (7) those who have severe systemic diseases and cannot tolerate examination.
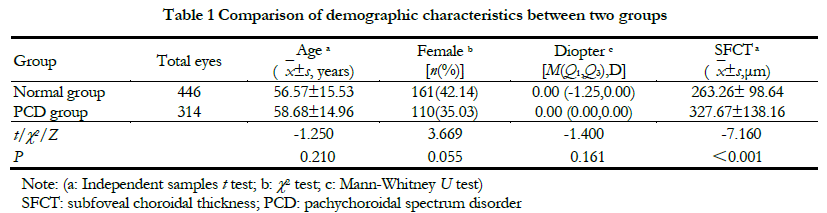
There were no significant differences in age, percentage of female and diopter between the two groups (all P>0.05). The subfoveal choroidal thickness (SFCT) value in the PCD group was greater than that in the normal group, and the difference was statistically significant (t=7.160, P<0.001) (Table 1). This study followed the Declaration of Helsinki, and the research protocol was approved by the Ethics Committee of Beijing Tongren Hospital (document No. TRECKY2016-054).
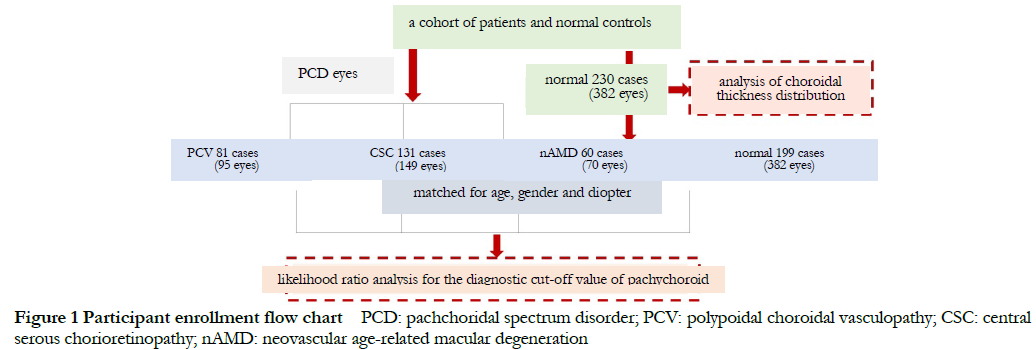
1.2 Subjects and Methods
1.2.1 Routine eye examination. The subjects underwent a comprehensive eye examination, including the best corrected visual acuity (BCVA); the intraocular pressure; anterior segment examination with slit lamp microscope (SL-IE, Japan Topcon Co., Ltd.); fundus examination under the dilated pupil (Santen Pharmaceutical Co., Ltd., Japan) with a binocular indirect ophthalmoscope (Keeler, USA), color photography using CR-1 non-dilation color fundus camera (Canon, Japan).
1.2.2 Measurement of macular choroidal thickness. All the enrolled subjects were examined by SS-OCT (DRI OCT-1 Atlantis scanner, Topcon, Japan) with a 9 mm×9 mm scanning range, and 12 high-resolution B-scan images which passed through the fovea were obtained and automatically analyzed by Topcon Advanced Boundary Segmentation (TABS) software in ring area around the fovea according to the Early Treatment Diabetic Retinopathy Study (ETDRS) classification regions. The radius of each eccentric ring was 1, 3 and 6 mm in turn, respectively. The value of choroidal thickness in the macula was automatically extracted, and the measurements were independently corrected manually by 2 senior ophthalmologists. The EDTRs scanning area was divided into 9 regions including the central area (C), upper inner ring area (S1), upper outer ring area (S2), nasal inner ring area (N1), nasal outer ring area (N2), and the lower inner ring area (I1), inferior outer ring area (I2), temporal inner ring area (T1), and temporal outer ring area (T2). The average value in zone C was selected for likelihood analysis.
1.2.3 Likelihood ratio test for determination of pachychoroid. Currently, there is still no precise diagnostic criteria of “pachychoroid” or “non-pachy choroid”, in this study, we applied likelihood ratio test to establish the diagnostic criteria. The subjects were divided into 20-39-year-old group, 40-59-year-old group, 60-79-year-old group and ≥80-year-old group in a 20-year-old intervals, and the choroidal thickness of all subjects was stratified by 100 μm from the minimum to the maximum value. The positive likelihood ratios were calculated based on the number of normal and diseased groups in different measurement value intervals. Positive likelihood ratio=(the number of eyes of each target measured interval of PCD group/total eyes number in PCD group)/(the number of eyes in target measured interval of normal group/total number of eyes in normal group). The interval of choroidal thickness values with a positive likelihood ratio close to 1 represented the critical value for distinguishing the choroidal thickness of the normal group and PCD group, and the choroidal thickness within this range was further stratified in units of 50, 25, and 10 μm to determine the diagnostic criteria (cut-off value) for pachychoroid after controlling for age and diopter.
1.2.4 The primary outcome measures. (1) Distribution characteristics of the choroidal thickness in normal eyes with age, diopter and gender; (2) Influencing factors of SFCT in normal eyes; (3) Evaluation of choroidal thickness diagnostic cut-off value for pachychoroid by likelihood ratio test.
1.3 Statistical analysis
SPSS 25.0 statistical software (IBM, USA) was utilized for statistical analysis. The Shapiro-Wilk test was applied to evaluate the distribution characteristics of numerical data and described as means±standard deviation (mean±SD) for the data with normal distribution or median (interquartile range, IQR) for the data of skewed distribution and the homogeneity of variance was confirmed by the Levene test between the groups. The differences in age, SFCT, SFCT between normal and PCD groups were compared by independent samples t test; the overall differences among the means of the factors were compared by one-way analysis of variance, and LSD-t test was used for post hoc analysis. The Mann-Whitney U test was used to compare the diopter differences between the two groups; the overall differences for skewness distributed data among groups were compared by Kruskal-Wallis H test; and Nemenyi test was used for post hoc test. Categorical data were described as frequency and percentage, and the difference between groups was compared by Chi-square test. Pearson linear correlation analysis was used to evaluate the relationship between age and SFCT in normal population, and Spearman rank correlation analysis was used to evaluate the relationship between diopter and SFCT in normal group. A multiple linear regression model was established to evaluate the influence of diopter, age, and gender to SFCT in normal people, in which gender was assigned as 1 in female and, men were assigned as 0 in male, and hypothesis test was performed on the regression equation. The likelihood ratio test was used to determine the diagnostic cut-off value of SFCT between normal control group and PCD group for every age range. A P value of 0.05 indicated statistical significance for all analyses.
2 Results
2.1 Correlation between age and SFCT in normal people and comparison of SFCT between different gender
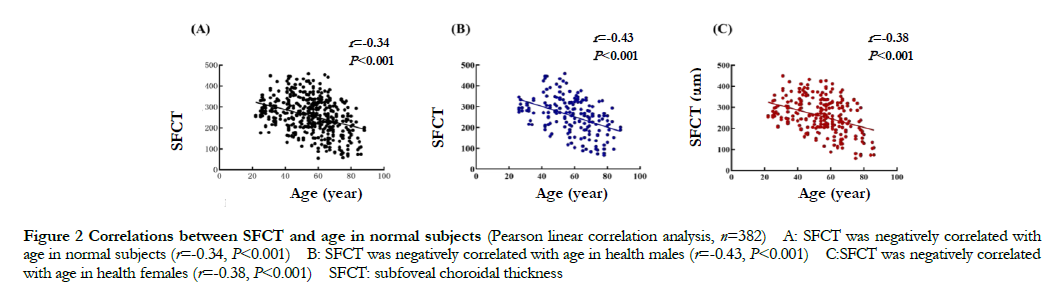
In normal people, the SFCT value was gradually reduced with the increase of age, showing a negatively correlated with age (r=-0.34, P<0.001). The SFCT values either in male or female in normal group were negatively correlated with age (r=-0.43, P<0.001; r=-0.38, P<0.001) (Fig. 2). The SFCT value was (257.04±86.23) μm in normal males, which was smaller than that of normal females (259.93±82.06) μm, but the difference was not statistically significant (t=-0.359, P>0.05).
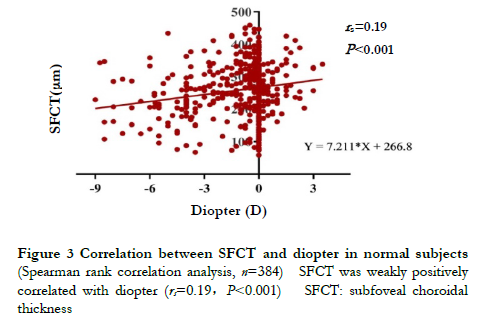
2.2 Correlation between diopter and SFCT in normal subjectsThere was a weak positive correlation between SFCT and diopter in normal subjects, and the SFCT value gradually increased with the increase of diopter (rs=0.19, P<0.001) (Fig.3).
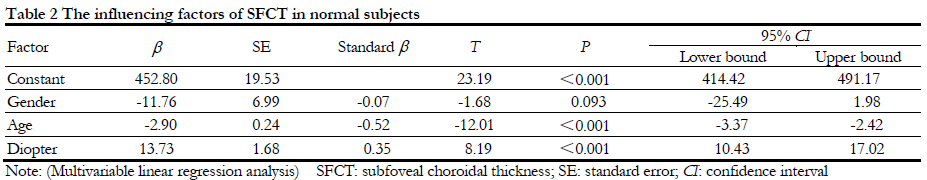
2.3 Influencing factors of SFCT
When SFCT was considered as the dependent variable, the age, gender and diopter were as independent variables, a multiple linear regression model showed age (t=-12.01, P<0.001; 95%CI:-3.37- -2.42) and diopter (t=8.19, P<0.001; 95%CI:10.43-17.02) were independent influencing factors of SFCT. After adjusted age and diopter, gender was not associated with SFCT (b=-11.76, 95%CI: -25.49-1.98, P=0.093) (Table 2).
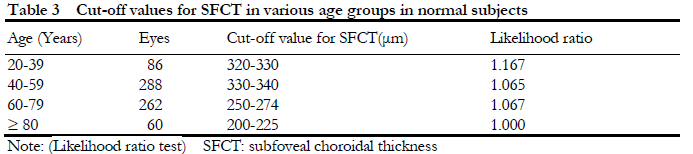
2.4 Diagnostic cut-off value for pachyoroid
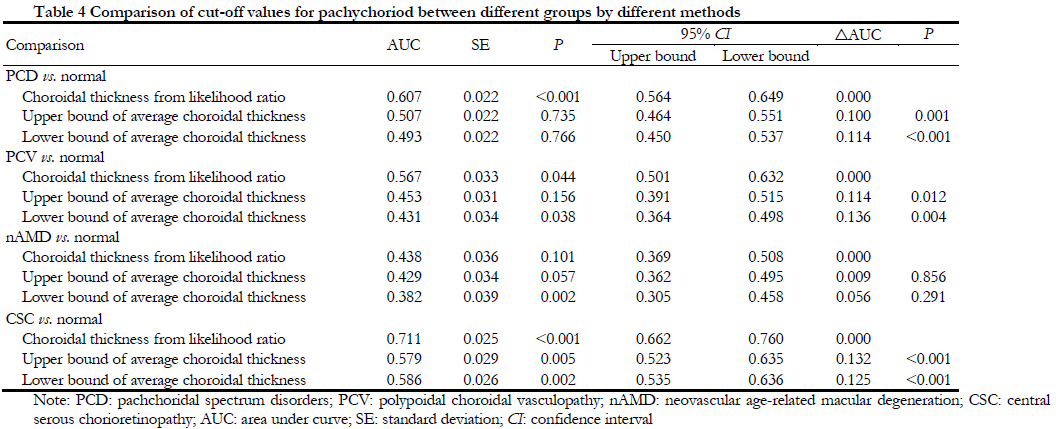
Likelihood ratio test showed that the SFCT value was gradually decreased with increased of the age. The SFCT likelihood ratios in different age groups were shown in Table 3. According to the diagnostic value determined in this study, the receiver operating curve (ROC) was used to estimate the area under the curve between the likelihood ratio test method and the current mainstream research method (mean±SD method). The result showed that the sensitivity and specificity of the likelihood ratio were significantly higher than mean±standard deviation method, especially in PCD vs. normal (P<0.001), CSC vs. Normal (P<0.001, and PCV vs. normal (P<0.044) (Table 4).
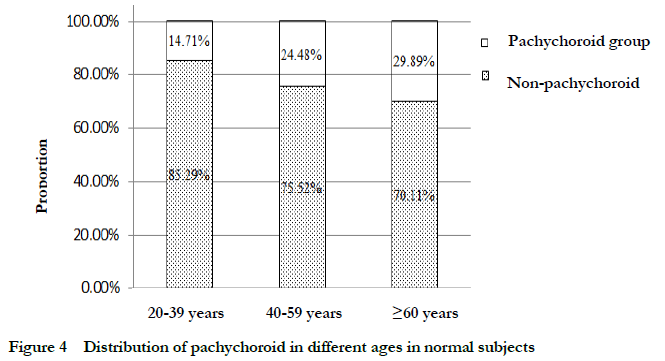
2.5 Comparison of the distribution of pachychoroid and non-pachychoroid in subjects of different ages in normal subjects
The percentage of pachychoroid in normal eyes was gradually increased with aging. The proportion of eyes with pachychoroid in 20-39 years old, 40-59 years old and ≥60 years old was 14.71% (10/68), 24.48% (47/192) and 28.89% (55/184), respectively,
showing an overall significant difference among groups (c2=6.170, P=0.046; LR=6.579, P=0.037), and the proportion of pachychoroid in ≥60-year-old group was significantly higher than that in 20-39-year-old group, and the difference was statistically significant (c2=5.982, P=0.014; LR=6.479, P=0.011) (Table 5, Fig. 4).
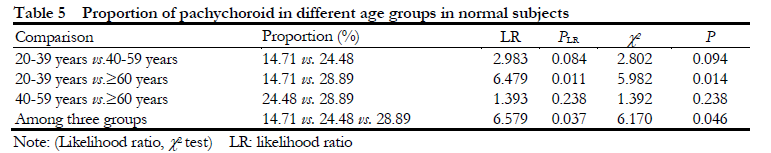
3 Discussion
In this study, the likelihood ratio test is utilized to define the diagnostic value of pachychoroid in Chinese normal subjects, and this is the first study which considers the influence of diopter, age and gender to pachychoroid. It is also found that age and diopter are negatively correlated with choroidal thickness. After adjusting for other influencing factors, gender is not associated with SFCT in this study. In addition, it is also found that the proportion of pachychoroid in different ages is significantly different after adjusting for age and diopter. It is interesting to further test whether pachychoroid in normal people is correlated with PCD and what are the correlated risk factors.
As one of the biomarkers for objective evaluation of the choroid, choroidal thickness depends on physiological and pathological factors of the body, and varies with age, diopter, axial length or circadian rhythm, and significantly individual differences are reported previously[9-13]. Under normal physiological conditions, depending on whether retinal pigment epithelium (RPE) detachment occurs, choroidal thickness is defined as the vertical distance from Bruch’s membrane hyperreflective line to the scleral inner hyperreflective line or the RPE hyperreflective line to the scleral inner layer reflection[14]. In addition, discrepancy is found in choroidal thickness measurement in normal subjects by using different instruments. Manjunath et al[15] measure the choroidal thickness and found SFCT value is slightly higher using SS-OCT (DRI-OCT Triton plus, Topcon) than that measured by spectral-domain OCT (SD-OCT), this result is consistent with Kim’s[16] and Ikuno[17] , they conclude that SS-OCT can be better in displaying the choroid-sclera interface than SD-OCT. Compared with SD-OCT, SS-OCT has a light source with a longer wavelength (central wavelength of 1 050/1 060 nm) and a double-balanced photodetector detection method, which has stronger penetrating ability and is affected by the light scattering of the RPE layer and the lens. The effect of turbidity and the signal attenuation is less, allowing better detection of signals from deeper layers[18-19]. The scanning depth of SS-OCT can reach 2.6 mm, and the maximum A scan can be obtained 400 000 times per second, and the axial resolution reaches 6.3 μm. By using SS-OCT, it is an ideal to obtain choroidal thickness with higher resolution of the choroid and sclera. In this study, we also control the most influencing factors for choroidal thickness including age and diopter in normal subject, in the future, more confounding factors need to be considered to explore more objective method and result for choroidal thickness measurement.
In this study, the multiple linear regression analysis model is utilized in the study to evaluate the associations between the confounding factors and SFCT. The results suggest that the SFCT in normal subjects is associated with age, gender and diopter, which is consistent with previous studies. Manjunath et al[15] study 34 normal subjects with an average age of 51.1 years and find that the average SFCT is moderately negatively correlated with age.
Relevant studies in Korea and Japan have also confirmed that age is the main influencing factor of choroidal thickness, follow by diopter[16-17]. Therefore, the influence of age, gender, and diopter or axial length on choroidal thickness should be considered in the analysis of SFCT.
At present, there is no a unified and standard method of measurement for choroidal thickness by OCT, and it is mainly divided into manual single-point or multi-point measurement and automatic segmentation method. The definition of pachychoroid varies from study to study due to different measurement methods. In order to reduce the measurement error, this study adopts a more objective automatic segmentation method, that is, the choroid layer is automatically partitioned according to the ETDRS standard using the built-in software of Topcon SS-OCT. The ETDRS map divides the macula into a central area, an inner ring, and an outer ring based on the radius of 1, 3, and 6 mm from the fovea, and further divides the inner and outer rings into 4 quadrants (superior, inferior, nasal, and temporal)[20-22]. By manual correction, the error caused by single-point measurement can be effectively reduced[5]. This study we mainly analyze the mean value of SFCT in the central area which is commonly used internationally.
There is no high-sensitivity and high-specificity evaluation method or gold standard for defining pachyhoroid. Dansingani et al[23] enrolled 66 eyes of 33 cases with PCD, defined the cutoff value for pachychoroid is SFCT ≥300 μm or the thickness of the thickest choroid at least 50 μm greater than SFCT. In addition, other major methods for quantifying standard threshold for pachychoroid include using the median thickness of PCV eyes as a classification criterion using the mean choroidal thickness plus standard deviation, or establishing a ROC to determine the cut off value in eyes with PCV[6-7,24-26]. However, these studies lack the comparison with normal human choroid thickness, and there is still no gold standard for the diagnosis of pachychoroid, the objective and quantitative criteria of pachychoroid is challenging. Recently, Spaide et al[27] used choriocapillary parameters by SS-OCT angiography in normal subjects, PCD patients, and pachychoroid groups to define the pachychoroid. Pachychoroid in normal eyes is defined as choroidal thickness ≥5% in the upper percentage of their age in the enrolled subjects. The choroidal thickness of the percentile subjects is simply expressed as: pachychoroid 5%ile≥525-3.25(age). However, none of the above methods consider the influence of factors of the choroidal thickness such as age, gender, and diopter, nor the sensitivity and specificity of critical diagnostic values. Since age is the most important factor affecting choroidal thickness, this study apply likelihood ratio test to compare and analyze the age stratified SFCT and PCD eyes in normal subjects, controlling for the influence of diopter, and to control the effect of diopter quantitatively to determine the cut-off value of SFCT. Through the validation in the population, it was found that the comparison of choroidal thickness is more significant using the likelihood ratio evaluation method than the mean±standard deviation evaluation method which are currently used more common.
Sensitivity and specificity are two important evaluation indicators of clinical diagnostic efficacy. The likelihood ratio test adjusts the confounding factors of age and diopter to determine the cutoff value of pachychoroid. In addition, the likelihood ratio combines the two indicators of sensitivity and specificity, which has better clinical significance[28].
Although the effects of diopter and gender on choroidal thickness are considered in this study, due to insufficient sample size, we do not find a correlation between SFCT and diopter and gender. More detailed multi-factor stratification is limited carried out in this study, the results from this study still need to be further verified in a cohort study with a larger sample.
In conclusion, this study selects the most advanced SS-OCT to obtain choroidal B-scan images after considering various influencing factors, we also use OCT’s software for automatic segmentation and manual correction to reduce measurement errors to obtain the choroidal thickness. As to the critical clinical significance of SFCT in normal people, a more objective quantitative diagnostic standard for pachychoroid has been proposed in this study. The normative definition of pachychoroid is not only helpful for the diagnosis and classification of PCD, but also crucial for further elaborating the pathogenesis of PCD and guiding treatment in the future.
Conflicts of Interest: None declared
References
[1] Zhang X, Sivaprasad S. Drusen and pachydrusen: the definition, pathogenesis, and clinical significance[J]. Eye (Lond), 2021, 35(1):121-133. DOI: 10.1038/s41433-020-01265-4.
[2] Zhang XY, Lai XY. Paying attention to the concept and research of pachychoroid disease spectrum[J]. Chin J Exp Ophthalmol, 2017, 35(5):385-390. DOI: 10.3760/cma.j.issn.2095-0160.2017.05.001.
[3] Coleman DJ, Lizzi FL. In vivo choroidal thickness measurement[J]. Am J Ophthalmol, 1979, 88(3 Pt 1):369-375. DOI: 10.1016/0002-9394(79)90635-4.
[4] Hillenkamp J, Hussain AA, Jackson TL, et al. The influence of path length and matrix components on ageing characteristics of transport between the choroid and the outer retina[J]. Invest Ophthalmol Vis Sci, 2004, 45(5):1493-1498. DOI: 10.1167/iovs.03-0765.
[5] Xie R, Qiu B, Chhablani J, et al. Evaluation of choroidal thickness using optical coherent tomography: a review[J/OL]. Front Med (Lausanne), 2021, 8:783519[2022-03-02]. https://pubmed.ncbi.nlm.nih.gov/34926529/. DOI: 10.3389/fmed.2021.783519.
[6] Gupta P, Ting D, Thakku SG, et al. Detailed characterization of choroidal morphologic and vascular features in age-related macular degeneration and polypoidal choroidal vasculopathy[J]. Retina, 2017, 37(12):2269-2280. DOI: 10.1097/IAE.0000000000001481.
[7] Liu ZY, Li B, Xia S, et al. Analysis of choroidal morphology and comparison of imaging findings of subtypes of polypoidal choroidal vasculopathy: a new classification system[J]. Int J Ophthalmol, 2020, 13(5):731-736. DOI: 10.18240/ijo.2020.05.06.
[8] Cheung C, Lai T, Teo K, et al. Polypoidal choroidal vasculopathy: consensus nomenclature and non-indocyanine green angiograph diagnostic criteria from the Asia-Pacific Ocular Imaging Society PCV workgroup[J]. Ophthalmology, 2021, 128(3):443-452. DOI: 10.1016/j.ophtha.2020.08.006.
[9] Spaide RF, Jaffe GJ, Sarraf D, et al. Consensus nomenclature for reporting neovascular age-related macular degeneration data: consensus on neovascular age-related macular degeneration nomenclature study group[J]. Ophthalmology, 2020, 127(5):616-636. DOI: 10.1016/j.ophtha.2019.11.004.
[10] Cheung C, Yanagi Y, Akiba M, et al. Improved detection and diagnosis of polypoidal choroidal vasculopathy using a combination of optical coherence tomography and optical coherence tomography angiography[J]. Retina, 2019, 39(9):1655-1663. DOI: 10.1097/IAE.0000000000002228.
[11] Iijima H, Iida T, Imai M, et al. Optical coherence tomography of orange-red subretinal lesions in eyes with idiopathic polypoidal choroidal vasculopathy[J]. Am J Ophthalmol, 2000, 129(1):21-26. DOI: 10.1016/s0002-9394(99)00253-6.
[12] Sanchez-Cano A, Orduna E, Segura F, et al. Choroidal thickness and volume in healthy young white adults and the relationships between them and axial length, ammetropy and sex[J]. Am J Ophthalmol, 2014, 158(3):574-583. DOI: 10.1016/j.ajo.2014.05.035.
[13] Nickla DL, Wallman J. The multifunctional choroid[J]. Prog Retin Eye Res, 2010, 29(2):144-168. DOI: 10.1016/j.preteyeres.2009.12.002.
[14] Moult EM, Waheed NK, Novais EA, et al. Swept-source optical coherence tomography angiography reveals choriocapillaris alterations in eyes with nascent geographic atrophy and drusen-associated geographic atrophy[J]. Retina, 2016, 36 Suppl 1:S2-S11. DOI: 10.1097/IAE.0000000000001287.
[15] Manjunath V, Taha M, Fujimoto JG, et al. Choroidal thickness in normal eyes measured using Cirrus HD optical coherence tomography[J]. Am J Ophthalmol, 2010, 150(3):325-329. DOI: 10.1016/j.ajo.2010.04.018.
[16] Kim M, Kim SS, Koh HJ, et al. Choroidal thickness, age, and refractive error in healthy Korean subjects[J]. Optom Vis Sci, 2014, 91(5):491-496. DOI: 10. 1097/OPX.0000000000000229.
[17] Ikuno Y, Kawaguchi K, Nouchi T, et al. Choroidal thickness in healthy Japanese subjects[J]. Invest Ophthalmol Vis Sci, 2010, 51(4):2173-2176. DOI: 10.1167/iovs.09-4383.
[18] Shin JW, Shin YU, Lee BR. Choroidal thickness and volume mapping by a six radial scan protocol on spectral-domain optical coherence tomography[J]. Ophthalmology, 2012, 119(5):1017-1023. DOI: 10.1016/j.ophtha.2011.10.029.
[19] Lim HB, Kim K, Won YK, et al. A comparison of choroidal thicknesses between pachychoroid and normochoroid eyes acquired from wide-field swept-source OCT[J/OL]. Acta Ophthalmol, 2021, 99(1):e117-e123[2022-03-02]. https://pubmed.ncbi. nlm.nih.gov/32573109/. DOI: 10.1111/aos.14522.
[20] Jirarattanasopa P, Ooto S, Tsujikawa A, et al. Assessment of macular choroidal thickness by optical coherence tomography and angiographic changes in central serous chorioretinopathy[J]. Ophthalmology, 2012, 119(8):1666-1678. DOI: 10.1016/j.ophtha.2012.02.021.
[21] Ruiz-Moreno JM, Flores-Moreno I, Lugo F, et al. Macular choroidal thickness in normal pediatric population measured by swept-source optical coherence tomography[J]. Invest Ophthalmol Vis Sci, 2013, 54(1):353-359. DOI: 10.1167/iovs.12-10863.
[22] Hirata M, Tsujikawa A, Matsumoto A, et al. Macular choroidal thickness and volume in normal subjects measured by swept-source optical coherence tomography[J]. Invest Ophthalmol Vis Sci, 2011, 52(8):4971-4978. DOI: 10.1167/iovs.11-7729.
[23] Dansingani KK, Balaratnasingam C, Naysan J, et al. En face imaging of pachychoroid spectrum disorders with swept-source optical coherence tomography[J]. Retina, 2016, 36(3):499-516. DOI: 10.1097/IAE.0000000000000742.
[24] Venkatesh R, Gadde S, Pereira A, et al. Impact of sub-foveal choroidal thickness on clinical features and long-term clinical outcomes in polypoidal choroidal vasculopathy[J]. Int Ophthalmol, 2021, 41(1):87-97. DOI: 10.1007/s10792-020- 01555-6.
[25] Kong M, Kim SM, Ham DI. Comparison of clinical features and 3-month treatment response among three different choroidal thickness groups in polypoidal choroidal vasculopathy[J/OL]. PLoS One, 2017, 12(9):e0184058 [2022-03-05].https://pubmed.ncbi.nlm.nih.gov/28886052/. DOI: 10.1371/journal. pone.0184058.
[26] Chang YC, Cheng CK. Difference between pachychoroid and nonpachychoroid polypoidal choroidal vasculopathy and their response to anti-vascular endothelial growth factor therapy[J]. Retina, 2020, 40(7):1403-1411. DOI: 10.1097/IAE. 0000000000002583.
[27] Spaide RF, Ledesma-Gil G. Choriocapillaris vascular parameters in normal eyes and those with pachychoroid with and without disease[J]. Retina, 2021, 41(4):679-685. DOI: 10.1097/IAE.0000000000002988.
[28] Hegedus EJ, Stern B. Beyond SpPIN and SnNOUT: considerations with dichotomous tests during assessment of diagnostic accuracy[J/OL]. J Man Manip Ther, 2009, 17(1):E1-E5[2022-03-05]. https://pubmed.ncbi.nlm.nih.gov/20046556/. DOI: 10.1179/jmt.2009.17.1.1E.