·Clinical Research·
Evaluation of efficacy and safety of phacoemulsification combined with gonioscopy-assessed angle plasty for primary angle closure glaucomas with cataracts
Wang Jin, Mou Dapeng, Zhang Ye, Wang Yue, Sun Yunxiao, Tang Xin, Wang Ningli
Beijing Ophthalmology & Visual Science Key Laboratory, Beijing Institute of Ophthalmology, Beijing Tongren Eye Center, Beijing Tongren Hospital, Capital Medical University, Beijing 100730,China
Corresponding author: Wang Ningli, Email:wningli@vip.163.com
[Abstract] [Download PDF in English] [Download PDF in Chinese] [Read Full Text]
Objective To evaluate the efficacy and safety of phacoemulsification combined with gonioscopy-assessed angle plasty (Phaco-GAAP) for primary angle closure glaucomas (PACGs) with cataracts.
Methods A case series study was carried out. Twenty-five eyes of 22 patients with PACGs and cataracts were enrolled at Beijing Tongren Hospital from April 2022 to August 2022. All patients received Phaco-GAAP surgery. During surgery, viscoelastic-assisted angle plasty was first performed, followed by a secondary angle plasty for residual peripheral anterior synechiae (PAS) based on the quantified assessment of gonioscopy, and the extent of PAS was recorded intraoperatively. The operated eyes were followed-up at 1 day, 1 week, 1 month, and 3 months after surgery to evaluate intraocular pressure (IOP), PAS range, the number of anti-glaucoma drugs used, operation-related complications, and success percentages. The qualified success percentage was defined as medicine-controlled IOP ≤ 21 mmHg after surgery, and complete success percentage was defined as IOP ≤ 21 mmHg without any anti-glaucoma medication.
Results The extent of PAS was [270(225,360)°] and [165(110,215)°]和[100(35,175)°] at presurgery, and at first and secondary angle plasty, respectively, showing a significant difference (χ2 = 40.742, P < 0.001). The PAS range was significantly reduced at first angle plasty in comparison with presurgery, at secondary angle plasty in comparison with at the first angle plasty (both, P < 0.001), and the percentage of angle PAS range ≥ 180° decreased from 48% to 24% after two angle plasties. In 13 eyes that finished gonioscopy, the PAS ranges were [240(195,305)°], [60(25,182.5)°], [170(120,275)°], and [180(140,280)°] at presurgery, intraoperative surgery, and postoperative surgery at 1 month and 3 months, respectively, with a significant difference among different time points (χ2 = 23.631, P < 0.001), the PAS range was significantly smaller at postoperative 1 month and 3 months than that at preoperation (P = 0.004), and larger than that at intraoperative PAS (P = 0.011 and P = 0.003, respectively). The IOPs were (40.19 ± 17.23), (15.80 ± 7.98), (13.89 ± 5.09), (12.80 ± 3.79), and (13.24 ± 2.78) mmHg before surgery and at 1 day, 1 week, 1 month, and 3 months after surgery, respectively, showing a significant difference among different time points (F = 44.031, P < 0.001), and the IOP was significantly reduced after surgery. The complete and qualified success percentages for 1 month and 3 months after surgery were 95.8%, 95.8%, 95.8%, and 100%, respectively. The primary complication was intraoperative anterior chamber angle hemorrhage, with an incidence of 68%.
Conclusions Gonioscopy-assisted phaco-GAAP intraoperatively quantified PAS range and guided secondary angle plasty. Therefore, it was an effective and safe surgical intervention for PACGs with cataracts.
[Key words] Primary angle closure glaucoma; Goniosynechialysis; Periphery anterior synechia; Outcome; Safety
Fund program: National Natural Science Foundation of China (82130029)
DOI: 10.3760/cma.j.cn115989-20221002-00465
Phacoemulsification combined with goniosynechialysis (Phaco-GSL) or phacoemulsification combined with viscogonioplasty (Phaco-VGP) is currently the surgical treatment of choice for primary angle closure glaucoma (PACG) patients in China 1. The pathogenesis of PACG is mainly due to the obstruction of aqueous humor discharge caused by angle closure, resulting in elevated intraocular pressure (IOP) and glaucomatous optic nerve damage 2. Previous studies have reported that simple lens extraction improved aqueous humor drainage by widening the chamber angle 3-4. However, for PACG patients with peripheral anterior synechiae (PAS) exceeding 180°, simple lens extraction has a limited effect for improving angle narrowing or adhesions 5, and long-term PAS can cause irreversible damage to trabecular tissues 6-7. Theoretically, lens extraction combined with goniosynechialysis (GSL) or gonioplasty is expected to relieve the resistance of aqueous humor drainage on the basis of improving the structure of the chamber angle, to directly treat the cause of the disorder. Therefore, how to improve traditional goniosynechialysis (GSL) or gonioplasty to effectively relieve the angle of adhesion to improve the therapeutic effects of PACG further has been a popular research topic.
GSL or gonioplasty are two different anti-glaucoma surgeries that open the angle, but there are certain differences in the surgical procedures. Internationally, the method of applying an iris separator or blunt instruments (such as viscoelastic needles, cataract chopper hooks, etc.) to mechanically compress the root of the iris to open the chamber angle with the assistance of a gonioscope is defined as GSL 8, while the method of directly injecting viscoelastic at the angle of the chamber and using the mechanical compression of the viscoelastic to separate the angle is defined as gonioplasty 9. At present, there is limited clinical research on gonioplasty in China. Studies have shown that the incidence of recurrent PAS after Phaco-GSL reached 83.2% 10. A retrospective study in 2022 showed that the incidence of recurrent PAS after supplementary mechanical GSL was higher, which may be related to factors such as mechanical damage to the angle of the chamber and/or trabecular meshwork, a bleeding tendency in the anterior chamber, and severe inflammatory responses. The study found that no matter whether the preoperative PAS range exceeded 180°, gonioplasty was achieved through viscoelastic filling, and more than half of the angles were open after surgery 11. However, how to achieve more effective intraoperative gonioplasty without increasing the difficulty and complexity of surgery, reduce mechanical damage caused by the separation of the angle as much as possible, and avoid extensive angle re-adhesion after surgery remains a challenge for ophthalmologists.
In the following report, our research team proposed a new surgical intervention method to effectively achieve VGP and gonioscopy-assessed angle plasty (GAAP). After phacoemulsification combined with intraocular lens (IOL) implantation, viscoelastic-assisted gonioplasty was first performed, the angle opening of 360° was evaluated with a gonioscope without adjusting the microscope, and the patient’s head position and supplementary angle plasty were performed on the incompletely opened angle. Overall, this study aimed to evaluate the efficacy and safety of Phaco-GAAP in the treatment of PACGs with cataracts.
1 Materials and methods
1.1 General information
This was an observational case series study. A total of 22 cases of 25 eyes with PACGs complicated with cataracts were enrolled at Beijing Tongren Hospital Affiliated with Capital Medical University from April 2022 to August 2022. The diagnostic criteria of PACGs were based on the International Society of Geographic and Epidemiologic Ophthalmology diagnostic criteria 12. There were four males and 18 females; the average age was (65.20 ± 10.36) years; the average cup/disk value of affected eyes was 0.65 ± 0.22; the average axial length was (22.21 ± 1.05) mm; and the mean defect value of the preoperative visual field was (-16.19 ± 14.34). Inclusion criteria were (1) age ≥ 40 years; (2) clinical diagnosis of PACG, preoperative dynamic gonioscopy PAS range ≥ 180°; (3) intraocular pressure or previous increase in intraocular pressure ≥ 21 mmHg (1 mmHg = 0.133 kPa), with glaucomatous optic nerve damage or visual field defects; and (4) with lens opacity, a best-corrected visual acuity ≤ 0.5 or decreased contrast sensitivity. Exclusion criteria were (1) a history of intraocular surgery or other invasive eye treatments (except laser peripheral iridectomy or argon ion laser peripheral iridoplasty); (2) secondary angle closure, uveitis, and angle-closure caused by neovascularization; (3) traumatic angle recession; (4) cataracts in an expansive phase, lens subluxation, or malignant glaucoma; (5) non-proliferative diabetic retinopathy, neovascularization or redness of the iris; (6) long-term history of local or systemic application of glucocorticoids; and (7) patients with severe systemic diseases who could not tolerate anti-glaucoma surgery.
The research protocol was reviewed and approved by the Ethics Committee of Beijing Tongren Hospital Affiliated to Capital Medical University (approval number: TRECKY2021-136). The research process complied with the Declaration of Helsinki, and all patients were aware of the research methods and purposes, and voluntarily signed the informed consent.
1.2 Methods
1.2.1 Preoperative examination All patients completed the preoperative examination. The visual acuity of the operated eye was measured using the international eye chart, the anterior segment of the patient was examined by a slit-lamp biomicroscope (Haag-Streit, Koniz, Switzerland), and the intraocular pressure was measured by a non-contact tonometer (CT-60; Topcon, Tokyo, Japan). A Humphrey 24⁃2 Perimeter (SITA Standard; Zeiss, Jena, Germany) was used to check the visual field of the affected eye, an ultrasonic biological microscope (50MH panoramic UBM; Shanghai Sower, Shanghai, China) was used for the examination, and an A/B type ultrasonic instrument (IOL Master 700; Zeiss) was used to measure IOL parameters, anterior chamber depth, lens thickness, and axial length.
1.2.2 Gonioscopy The same glaucoma specialist performed the gonioscopy. Static gonioscopy was performed with a single-mirror indentation gonioscope (Ocular Instruments Company, USA) with a 1 mm narrow beam without oppressing the eyeball and no obvious eyeball rotation. The angle width was graded according to the Scheie grading system. Dynamic gonioscopy was used to determine the PAS range of the room angle. PAS was defined as the peripheral iris occlusion function of the trabecular network, and the room angle was graded as narrow 3-4 (N3-N4).
1.2.3 Surgical methods The same surgeon performed all operations. During surgery, an upper or upper temporal clear corneal incision was made, viscoelastic was injected into the anterior chamber, circular capsulorhexis was performed, water separation was performed with a balanced salt solution, the lens nucleus was phacoemulsified, the lens cortex was aspirated with an aspiration (automated or manual) of cortical lens matter needle, and the viscoelastic agent was injected into the capsular bag with an implanted foldable IOL (250; Hoya, Tokyo, Japan). The viscoelastic agent was injected around the anterior chamber angle with a viscoelastic needle, and the first gonioplasty was performed. Intraoperative gonioscopy (Ahmed 1.3X Surgical Gonio Lens-H; Ocular Instruments Company, USA) was used to check the location and range of PAS, the second gonioplasty was performed on eyes with incomplete opening of the angle, and the PAS range was recorded after surgery and intraoperative complications (please scan the QR code to watch the operation video). If angle hemorrhage occurred during surgery, the viscoelastic agent was injected into the anterior chamber to increase the intraocular pressure for hemostasis. The viscoelastic agent, pigment, and hemorrhage were moved in the anterior chamber with an aspiration (automated or manual) of cortical lens matter needle and the incision was sealed with stromal hydration. If the angle closure exceeded 180°, the surgery was combined with endoscopic photocoagulation (ECP).
1.2.4 Postoperative treatments After surgery, tobramycin and dexamethasone eye drops were administered four times a day, and tobramycin and dexamethasone eye ointment was administered to the conjunctival sac once before sleeping, with pranoprofen eye drops being administered four times a day, for a total of 3 weeks. Anti-glaucoma drugs were discontinued after surgery. If the intraocular pressure of the postoperative eye increased ≥ 21 mmHg, one or two anti-glaucoma drugs were applied locally.
1.2.5 Follow-up and evaluation indicators Intraocular pressure measurement and anterior segment slit-lamp microscopy were performed 1 day, 1 week, 1 month, and 3 months after surgery, respectively. Gonioscopy was used at 1 month and 3 months after surgery. Successful surgery was defined as a postoperative intraocular pressure ≤ 21mmHg.
Conditional success was defined as a postoperative intraocular pressure that could be controlled at ≤ 21 mmHg, using an anti-glaucoma drug. Postoperative intraocular pressure ≤ 21 mmHg without intraocular pressure-lowering was defined as a complete success. Uncontrolled postoperative intraocular pressure with the need for another anti-glaucoma surgery was defined as failed surgery. The main outcome indicators were IOP decreases, PAS ranges at 1 and 3 months after surgery, and the secondary outcome indicators were the number of IOP-lowering drugs used, surgical success percentages, and surgical complications.
1.3 Statistical methods
Statistical analysis was performed using SPSS statistical software for Windows, version 26.0 (SPSS, Chicago, IL, USA). Shapiro-Wilk test was used to evaluate the normal distributions of measured data, and normal distribution of measurement data was represented by the ±s, and homogeneity of variances was confirmed by Mauchly’s test of sphericity. Repeated measured one-way analysis of variance was used to compare the overall difference of intraocular pressures at different times before and after surgery, α=0.05; Bonferroni test for pairwise comparison, α’=0.05/4 (0.013). The measurement data with non-normal distribution were represented by M (Q1, Q3), and the overall difference in PAS range of each operation eye was compared using the Friedman M test, α=0.05, and Bonferroni test was employed for pairwise comparisons, α’=0.05/4 (0.013). Spearman’s rank correlation analysis was used to identify the correlations the between preoperative PAS ranges and postoperative PAS ranges.
2 Results
2.1 Comparison of PAS ranges before and during surgery
There was a statistically significant difference in the overall PAS range between preoperative and intraoperative first and second gonioplasties (χ2 = 40.742, P < 0.001). The PAS range after the first and second gonioplasties was significantly smaller than that before surgery, and the PAS range after the second gonioplasty was significantly smaller than that after the first gonioplasty (all, P < 0.001) (Table 1). After the first gonioplasty, a PAS range ≥ 180° accounted for 48% (12/25), and decreased to 24% (6/25) after the second gonioplasty. After the second gonioplasty, if the angle of the chamber persisted and the adhesion closed ≥ 180°, ECP was combined during surgery, and the range of cyclophotocoagulation was 120º−180°, which was selected according to the preoperative intraocular pressure and visual field conditions of the operated eye. Spearman’s correlation analysis showed that there was a positive correlation between the postoperative and preoperative PAS ranges (rs = 0.409, P = 0.042).
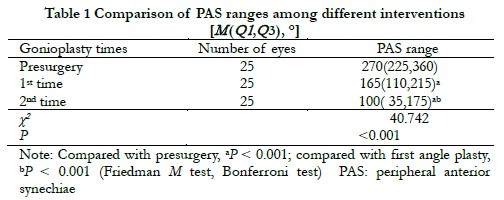
2.2 Comparison of the PAS ranges in operated eyes at different follow-up times
At the end of follow-up, gonioscopy was completed in 13 eyes. There was a statistically significant difference in PAS before surgery, after surgery, at 1 month after surgery, and at 3 months after surgery (χ2 = 23.631, P < 0.001). The PAS ranges of the operated eye at 1 month and 3 months were significantly smaller than those before surgery (both, P = 0.004), but was greater than the PAS range of the chamber angle at the end of surgery, with the differences being statistically significant (P = 0.011 and P = 0.003, respectively) (Table 2) .
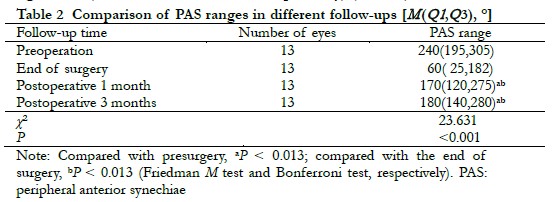
2.3 Comparison of intraocular pressures before and after surgery
The intraocular pressures of the eyes at preoperation, postoperative 1 day, postoperative 1 week, postoperative 1 month and postoperative 3 months were (40.19 ± 17.23), (15.80 ± 7.98), (13.89 ± 5.09), (12.80 ± 3.79), and (13.24 ± 2.78) mmHg, and there were a significant difference among different time points (F = 44.031, P < 0.001) (Figure 1).
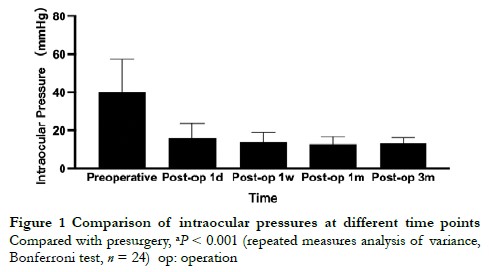
2.4 The use of anti-glaucoma drugs before and after surgery and the surgical success percentage
The average number of anti-glaucoma drugs used before surgery was 3.5 (3, 4), and the average numbers of drugs used 1 day, 1 week, 1 month, and 3 months after surgery were 0 (0, 0), 0 (0, 0), 0 (0, 0), 0 (0, 0), and one eye, two eyes, one eye, and one eye were treated with local anti-glaucoma drugs, respectively.
At 1 month after surgery, anti-glaucoma drugs were used to lower the intraocular pressure > 21 mmHg in one eye, and the complete success percentages of the surgical and conditional success percentages were both 95.83%; 3 months after surgery, the complete success percentage of surgery was 95.83%. For one eye with intraocular pressure < 21 mmHg using topical medication, the conditional success percentage was 100%.
2.5 Complications related to eye surgery and their treatments
The incidence of intraoperative anterior chamber angle hemorrhage was 68% (17/25), all of which were localized angle hemorrhage, and the bleeding stopped spontaneously after IA suction. Patients with vitreous hernia and Descemet’s membrane detachment during surgery comprised two eyes, respectively, with each accounting for 8.0%. There were five eyes with transient intraocular pressure > 21 mmHg at 1 day after surgery, which accounted for 20.0%, and two eyes with intraocular pressure > 21 mmHg after retesting 1 week after anterior chamber puncture, accounting for 8.0%. Local anti-glaucoma drugs were also used for treatment. Anterior chamber inflammation occurred in six eyes soon after surgery, accounting for 24%. A subconjunctival injection of dexamethasone was given once a day for 3 consecutive days, which alleviated inflammation. One quadrant of shallow ciliary detachment occurred 1 day after surgery in one eye, accounting for 4.0%. Anterior segment OCT showed ciliary detachment, which was relieved 1 month after surgery; corneal edema occurred in one eye 1 day after surgery, accounting for 4.0%. After 1 week, transparency was restored. No serious complications, such as a shallow anterior chamber, malignant glaucoma, and choroidal detachment, were found during the early postoperative period.
3 Discussion
This study was the first to quantitatively analyze changes of PAS during gonioplasty, and confirmed that Phaco-GAAP surgery significantly increased the opening range of the anterior angle intraoperatively, showing that it was a safe and effective surgical method for the treatment of PACGs with cataracts. Currently, there is still a lack of reports on the quantitative evaluation of intraoperative angle openings, which affects objective evaluation of the efficacy of PACG surgery. The gonioscope used in GAAP surgery does not need to adjust the operating microscope and the patient’s head position, and the whole circumference of the room angle can be observed by rotating the gonioscope. Compared with the gonioscope in GSL surgery, it was more convenient to observe the upper part, and at the same time, the learning period was short, and it was easy to promote. The reason for continuing to use a viscoelastic agent for supplementary angle separation instead of using an iris separator or blunt instrument for mechanical separation was that the viscoelastic agent resulted in less damage to the angle, and the time of PAS angle adhesion that could not be separated by the viscoelastic agent for the second time was longer and the adhesion was tighter. Prolonged angle closure can cause Schlemm canal endothelial cell damage and trabecular cell damage, reducing the function of the trabecular meshwork and affecting the discharge of aqueous humor 6. Therefore, even if the angle is forcibly separated, the function of the trabecular meshwork may not be restored, but it will increase the risk of intraoperative angle hemorrhage, aggravate the intraoperative inflammatory response, and cause mechanical damage to the surrounding functional trabecular meshwork.
Wei et al. 11 studied the influences of different preoperative PAS ranges on postoperative recurrent PAS, and found that the larger the preoperative angle adhesion range, the larger the postoperative recurrent PAS range. The present study found that there was also a positive correlation between the PAS range of the chamber angle at the end of surgery and the preoperative PAS range, suggesting that the preoperative PAS range affected the range of the chamber angle opening at the end of the operation, and also affected the postoperative recurrence percentage. Future research should continue to investigate whether intraocular pressure and disease severity can predict the degree of intraoperative angle opening, in addition to the PAS range of the preoperative angle, and should identify risk factors for postoperative angle re-adhesion, which should provide the basis for treatment of PACGs with cataracts using an angle separation technique.
For the eyes whose intraoperative chamber angle PAS range is always >180°, the selection of combined surgical methods has been rarely reported. The reason why the present study did not consider continuing to use gonioplasty or GSL to achieve full-circumference angle opening was that PAS with too tight adhesion will cause more damage to the trabecular meshwork after forcible separation, with the possibility of normal function of this part of the trabecular meshwork being relatively low. Excessive viscoelastic accumulation in the anterior chamber and chamber angle will also increase the risk of intraoperative and early postoperative transient high intraocular pressure and early postoperative ciliary detachment. Therefore, for these patients, the combined ECP treatment used in this study improved the non-pupil block factors such as ciliary body rotated anteriorly and hypertrophy 13, but also destroyed part of the ciliary process to reduce the secretion of aqueous humor. Angles that are edematous and cannot be assessed with a gonioscope can be easily visualized endoscopically. A case series study in 2019 confirmed that Phaco-VGP combined with ECP in the treatment of chronic angle-closure glaucoma, resulted in 72.4% of the patients having intraocular pressure controlled below 14 mmHg at 6 months after surgery 14.
In previous studies, Phaco-GSL or Phaco-VGP mostly used indicators such as intraocular pressure changes and the number of anti-glaucoma drugs, to define the success of surgery, so the results of different studies differed due to different inclusion criteria and follow-up times. Kameda et al. 15 evaluated the curative effect of Phaco-GSL at 1 and 3 years after surgery, with the success percentage being 85.9%. Harasymowycz et al. 14 found that 95% of eyes with acute PACG who received Phaco-GSL had intraocular pressures ≤ 21 mmHg. The complete success percentage was 57.9%, and the conditional success percentage was 92.1%. A randomized controlled clinical study by Angmo et al. 17 found that for drug-controlled PACG patients, the success of Phaco-VGP was 91.2% at 6 months after surgery. In the present study, complete success of surgery 3 months after surgery was 95.8%, which was close to the results of other short-term observation studies. The gradual closure of synechia of the angle may be manifested as an increase in fluctuations of intraocular pressure. Therefore, a single intraocular pressure measurement cannot truly reflect the control of intraocular pressure. The quantitative index of the postoperative angle adhesion range was added to evaluate angle-closure glaucoma surgery. It may be more objective to evaluate the success of surgery at different time points, and at the same time, it can also avoid the aggravation of glaucomatous optic nerve damage caused by chronic intraocular pressure elevation caused by occult angle closure in some patients.
This study also found that intraocular pressure of four patients with PAS was ≥ 270°at 3 months after surgery, which was controlled without topical application of anti-glaucoma drugs, suggesting that there was a minimum effective outflow range (MEOR) in patients with angle-closure glaucoma. The aqueous humor can therefore be discharged through the limited open area of the chamber angle to maintain stability of the intraocular pressure, so some patients could achieve dynamic balance of the production and discharge of the aqueous humor even if the open range of the anterior chamber angle was only 90°. It was related to the presence of a dominant drainage area for aqueous humor outflow, which needs further study. The direction of angle opening surgery in the future is therefore not to achieve full-circumference angle opening as much as possible, but rather to increase MEOR as much as possible.
According to previous reports, the incidence of localized anterior chamber hemorrhage during and after Phaco-GSL or Phaco-VGP was 10.4%−60% 18-20, and the incidence of anterior chamber inflammation was 19.2% 19. The incidence of transient elevated intraocular pressure was 13.2% 10. In the present study, the incidence of anterior chamber hemorrhage was slightly higher, which may have been related to factors such as multiple angle separations during surgery, intraocular pressure fluctuations during surgery, and blood backflow from the Schlemm canal into the anterior chamber. Bleeding was limited and could be stopped by itself. Whether there is a relationship between limited anterior chamber angle hemorrhage and postoperative progressive PAS remains to be confirmed by further studies. Postoperative transient high intraocular pressure occurred in some operated eyes, which was usually related to retention of the viscoelastic agent during surgery, especially viscoelastic agent in the angle of the chamber, which could be alleviated by anterior chamber puncture and drainage after surgery. In the present study, the six eyes with postoperative anterior chamber inflammatory reactions were all patients who underwent ECP during surgery. They showed a decrease after anti-inflammatory treatment, and no anterior chamber exudate membrane was formed; but whether the inflammatory reaction after ECP increases the postoperative angle, and if re-adhesion remains has yet to be determined. The intraoperative vitreous hernia may be related to relaxation of the zonular ligament and liquefaction of the vitreous body. In the present study, there were two eyes with localized Descemet’s membrane detachments during surgery, which recovered spontaneously after surgery, and which may be related to the patients’ severe cataract, the time spent using anterior chamber ultrasound, and too many operations. Small scale Descemet’s membrane detachment seen during surgery cannot be treated, and the large detachment can be left with air in the anterior chamber at the end of surgery to promote recovery. In the present study, shallow ciliary body detachment occurred early after surgery in one eye, which may have been caused by excessive penetration of the needle into the angle of the anterior chamber during surgery. Small-scale and shallow ciliary detachment can usually recover on their own. If the scale is large or there is too much fluid in the supraciliary cavity, it may cause the ciliary body to rotate forward and push the peripheral iris, causing the angle to close. OCT of the anterior segment should therefore be used to evaluate the state of the ciliary body during the early postoperative period. If the scope of ciliary body detachment is large, anti-inflammatory medications should be increased to prevent permanent PAS caused by long-term ciliary body detachment.
The innovation of this study was that it was the first to quantitatively evaluate the degree of angle opening in combined glaucoma and cataract surgeries. Downward secondary gonioplasty can effectively expand the opening range of the intraoperative chamber angle. At the same time, compared with GSL under direct vision of the gonioscope, the surgical method in this study was safer, had less damage to the trabecular meshwork, and did not increase the difficulty and duration of the operation. In view of the high incidence and blindness from angle-closure glaucoma in China, this study provided a new surgical method for further improvement of angle separation surgery, so it effectively improved the curative effect of PACG combined with cataract surgery. The surgical method provided in this study is highly operable and has good reference value for clinical work. The limitation of this study was mainly that it was an observational series of cases, the included sample size was small, and the follow-up time was only 3 months. Therefore, the results of this study still need to be verified by randomized controlled clinical studies with good designs, large sample sizes, and long-term follow-ups.
Conflict of interest All authors declare no conflict of interest.
Author Contribution Statement Wang Jin: topic selection, research implementation, data collection and analysis, and paper writing; Mou Dapeng, Zhang Ye, Wang Yue, Sun Yunxiao, and Tang Xin: research implementation, data collection, and paper revision; Wang Ningli topic selection, research implementation, revision of the intellectual content of the thesis, and finalization.
References
[1] Glaucoma Research Group, Ophthalmology Branch of Chinese Medical Association. Expert consensus on the diagnosis and treatment of primary angle-closure glaucoma in China (2019)[J]. Chin J Ophthalmol, 2019, 55(5):325-328. DOI:10.3760/cma.j.issn.0412-4081.2019.05.002
[2] Yanoff, Myron, and Jay S. Duker. Ophthalmology[M]. 5th ed. Amsterdam:Elsevier, 2018:1064
[3] Zheng DJ, Wang NL, Zhen ZZ, et al. The change of configuration about anterior chamber angle of primary angle-closure glaucoma before and after extraction of cataract[J]. Ophthalmol CHN, 2000, 9(3):131-135. DOI: 10.3969/j.issn.1004-4469.2000.03.001.
[4] Liang YB, Wang NL, Qiao LY, et al. Assessment of a simple cataract extraction for the treatment of angle-closure glaucoma coexisting with cataract [J]. Chin J Ophthalmol, 2004, 40(11):3. DOI: 10.3760/ j:issn:0412-4081.2004.11.002.
[5] Krishnadas R. Current management options in primary angle closure disease[J]. Indian J Ophthalmol, 2019, 67(3):321-323. DOI: 10.4103/ijo.IJO_1932_18.
[6] Hamanaka T, Kasahara K, Takemura T. Histopathology of the trabecular meshwork and Schlemm’s canal in primary angle-closure glaucoma[J]. Invest Ophthalmol Vis Sci, 2011, 52(12):8849-8861. DOI: 10.1167/iovs.11-7591.
[7] Sihota R, Goyal A, Kaur J, et al. Scanning electron microscopy of the trabecular meshwork: understanding the pathogenesis of primary angle closure glaucoma[J]. Indian J Ophthalmol, 2012, 60(3):183-188. DOI:10.4103/0301-4738.95868.
[8] Campbell DG, Vela A. Modern goniosynechialysis for the treatment of synechial angle-closure glaucoma[J]. Ophthalmology, 1984, 91(9):1052-1060. DOI:10. 1016/s0161-6420(84)34195-1.
[9] Varma D, Adams WE, Phelan PS, et al. Viscogonioplasty in patients with chronic narrow angle glaucoma[J]. Br J Ophthalmol, 2006, 90(5):648-649. DOI:10. 1136/bjo.2005.087577.
[10] Tian T, Li M, Pan Y, et al. The effect of phacoemulsification plus goniosynechialysis in acute and chronic angle closure patients with extensive goniosynechiae[J/OL]. BMC Ophthalmol, 2019, 19(1):65[2022-09-20]. http://pubmed.ncbi.nlm.nih.gov/30832600. DOI: 10.1186/s12886- 019-1070-9.
[11]Wei L, Fu L, Nie L, et al. Efficacy of combined phacoemulsification and goniosynechialysis in primary angle closure disease with different degrees of peripheral anterior synechiae[J]. J Glaucoma, 2022, 31(7):540-546. DOI: 10.1097/IJG.0000000000002050.
[12] Foster PJ, Buhrmann R, Quigley HA, et al. The definition and classification of glaucoma in prevalence surveys[J]. Br J Ophthalmol, 2002, 86(2):238-242. DOI: 10.1136/bjo.86.2.238.
[13] Villavicencio JCI, Arbelaez NA, Lastra BR, et al. Primary outcomes of patients with chronic angle-closure glaucoma treated with combined phacoemulsification, viscogoniosynechialysis, and endocyclophotocoagulation[J/OL]. J Ophthalmol, 2019, 2019:6378489[2022-09-20]. https://pubmed.ncbi.nlm.nih.gov/31312531/. DOI: 10.1155/2019/6378489.
[14] Harasymowycz PJ, Papamatheakis DG, Ahmed I, et al. Phacoemulsification and goniosynechialysis in the management of unresponsive primary angle closure[J]. J Glaucoma, 2005, 14(3):186-189. DOI: 10.1097/01.ijg.0000159131.38828.85.
[15] Kameda T, Inoue T, Inatani M, et al. Long-term efficacy of goniosynechialysis combined with phacoemulsification for primary angle closure[J]. Graefe´s Arch Clin Exp Ophthalmol, 2013, 251:825-830. DOI: 10.1007/s00417-012-2091-8.
[16] Husain R, Do T, Lai J, et al. Efficacy of phacoemulsification alone vs phacoemulsification with goniosynechialysis in patients with primary angle-closure disease: a randomized clinical trial[J]. JAMA Ophthalmol, 2019,137(10):1107-1113. DOI:10.1001/jamaophthal mol.2019.2493.
[17] Angmo D, Shakrawal J, Gupta B, et al. Comparative evaluation of phacoemulsification alone versus phacoemulsification with goniosynechialysis in primary angle-closure glaucoma: a randomized controlled trial[J]. Ophthalmol Glaucoma, 2019, 2(5):346-356. DOI: 10.1016/j.ogla.2019.05. 004.
[18] Nie L, Pan W, Fang A, et al. Combined phacoemulsification and goniosynechialysis under an endoscope for chronic primary angle-closure glaucoma[J/OL]. J Ophthalmol, 2018, 2018:8160184[2022-09-22]. https:// pubmed.ncbi.nlm.nih.gov/29576881/. DOI:10.1155/2018/8160184.
[19] Teekhasaenee C, Ritch R. Combined phacoemulsification and goniosynechialysis for uncontrolled chronic angle-closure glaucoma after acute angle-closure glaucoma[J]. Ophthalmology, 1999, 106(4):669-674, discussion 674-675. DOI: 10.1016/S0161-6420(99)90149-5.
[20] Chen Y, Cheng GW. A comparison study on goniosynechialysis+ phacoemulsification versus simple phacoemulsification for CPACG with PAS≤180° combined cataract[J]. Chin J Exp Ophthalmol, 2021, 39(10):885-891. DOI: 10.3760/ cma.j.cn115989-20201222-00857.