·Experimental Research·
Feasibility of corneal epithelial transplantation with polyethylene glycol hydrogel membrane as a carrier for limbal stem cell deficiency
Guo Yiyuan1, Xian Huimin2, Shereen Tan3, Qiang Fu3,4, Jin Xin1, Mark Daniel5, Greg.G.Qiao2, Zhang Hong1
1Department of Ophthalmology, The First Affiliated Hospital of Harbin Medical University, Harbin 150001, China; 2Department of Cardiology, The 2nd Affiliated Hospital of Harbin Medical University, Harbin 150001, China; 3Department of Chemical and Biomolecular Engineering, The University of Melbourne, Victoria 3001, Australia; 4The School of Civil and Environmental Engineering, University of Technology Sydney, Sydney 2000, Australia; 5Centre for Eye Research Australia (CERA), Royal Victorian Eye & Ear Hospital, Melbourne3002, Australia
Corresponding author: Zhang Hong, Email: zhanghong@hrbmu.edu.cn
[Abstract] [View PDF in English] [View PDF in Chinese] [Read Full Text]
Objective To investigate whether thyleneglycol hdrogel films(PHFs)can be used as a carrier for the expansion of corneal epithelial cells (CECs) in vitro and whether PHFs can be used in the treatment of limbal stem cell deficiency (LSCD).
Methods Sebacoyl chloride, dihydroxyl PCL and glycerol ethoxylate were used to synthesize PHFs. The thickness, transmittance and mechanical tensile properties of PHFs were measured. Four clean-grade New Zealand white rabbits were selected to culture primary limbal epithelial cells. The expression of keratin marker AE1/AE3 and stem cell marker p63 in the cultured cells were observed under a Fluorescence microscope. The cells were divided into negative control group cultured with common cell culture solution, positive control group cultured with cell culture solution containing 100 μmol/L H2O2, and PHFs+CECs group lined with PHFs cultured with common cell culture solution for 24 hours. The proliferation and apoptosis of cells in the three groups were observed by MTT and TUNEL staining, respectively. Fifteen clean-grade New Zealand white rabbits were divided into control group, PHFs group and PHFs+CECs group by random number table method, with 5 rabbits in each group. LSCD model was constructed in the three groups. The control group was not given any treatment after modeling. In PHFs group, empty PHFs were placed on the corneal surface of rabbits. In PHFs+CECsgroup, tissue-engineered grafts constructed with CECs after passage implanted on PHFs were placed on the corneal surface of rabbits. The corneal defect area of rabbits was detected and scored by fluorescein sodium staining. The histological characteristics of rabbits’ corneal epithelium were observed by hematoxylin-eosin staining. The use and care of animals complied with the Guide for the Care and Use of Laboratory Animals by the U.S. National Research Council. The experimental protocol was approved by the Research and Clinical Trial Ethics Committee of The First Affiliated Hospital of Harbin Medical University (No.2021006).
Results The synthetic PHFs were with a thickness of ≤150 μm, a tensile strength of about 6 MPa, and a transmittance over 99% in the range of 400-700 nm. Most of the cells from primary culture of limbal tissue were positive for AEl/AE3 and p63.MTT test results showed that the A490 values of PHFs+CECs group and negative control group and positive control group were 0.59±0.01, 0.65±0.07 and 0.06±0.04, respectively, showing a statistically significant overall
difference (F=12.25, P<0.05). The A490 values of PHFs+CECs group and negative control group were significantly higher than that of positive control group, and the differences were statistically significant (both at P<0.05).TUNEL test results showed that there was a significant difference in the TUNEL-positive cell rate among the three groups (F=13.45, P<0.05), and the rates of TUNEL-positive cells in PHFs+CECs group and negative control group were significantly lower than that in positive control group (both at P<0.05). Fluorescein sodium staining results showed that with the extension of the postoperative period, the corneal fluorescein sodium staining score of the three groups decreased, which decreased successively in control group, PHFs group and PHFs+CECs group. Hematoxylin-eosin staining showed fewer irregularly shaped corneal epithelial cells in the control group, and sparse single layer of corneal epithelial cells in some areas of the PHFs group. In PHFs+CECs group, the corneal epithelium coverage was the largest, the cell layers increased to 3-5, and the cells were with regular morphology and close arrangement.
Conclusions PHFs have enough toughness, high transmittance and can expand corneal epithelium in vitro. PHFs are suitable for corneal epithelial transplantation and can promote the repair of corneal epithelium in a rabbit model of LSCD.
[Key words] Epithelial cells, corneal; Limbal stem cell deficiency; Polyethylene glycol hydrogel film; Corneal epithelial cell regeneration; Corneal epithelial grafts
Fund program: National Natural Science Foundation of China (U20A20363, 81970776); Nature Scientific Foundation of Heilongjiang Province (LH2020H039); Science and Technology Innovation Base Award Project of Heilongjiang Province (JD22C006); Postdoctoral Program of Heilongjiang Province (LBH-Z22206)
DOI: 10.3760/cma.j.cn115989-20201125-00794
Limbal stem cell transplantation is an effective method to treat limbal stem cell deficiency (LSCD), but because of the limitation of autotransplantation and allograft rejection, the operation of transplantation is limited. Limbal stem cells (LSCs) can differentiate into corneal epithelial cells (CECs), but the injury of LSCs by operation, trauma and infection can cause LSCD, which results in the loss of corneal integrity and transparency, finally appearing corneal conjunctivitis, vascularization and other ocular surface chronic inflammation changes1-2. At present, transplantation of LSCs is considered an effective method to restore the reserve of LSCs, reconstruct the ocular surface and treat LSCD. Among them, there is no immune rejection in limbal stem cell autotransplantation, which has a high success rate, but it is only suitable for patients with small-scale injury of a single eye, and there are also patients with irreversible injury of the healthy eye caused by surgical operation. Allogeneic limbal stem cell transplantation is suitable for the treatment of extensive injury of both eyes, but there are some problems such as insufficient donor cornea, immune rejection, and so on3. In recent years, with the development of tissue engineering, the use of tissue engineering to amplify CECs in vitro and construct epithelial grafts provides a new approach for the treatment of LSCD, it can effectively solve the problems related to autotransplantation and allotransplantation of limbal stem cells, and provide a new way for the treatment of LSCD. The correct choice of carrier is crucial to the success of tissue engineering, at present, the CECS carriers in common use include amniotic membrane, silk fibroin membrane, collagen hydrogel, chitosan hydrogel, etc.4-5, these carriers have the disadvantages of poor transmittance, a great difference between donors, instability, poor mechanical strength and so on6-7, so it is necessary to find a suitable CECs tissue engineering carrier. Polyethyleneglycol hydrogel films (PHFs) have been proven to be a novel Polyethylene glycol carrier, which has the advantages of high transmittance, high toughness, low immunoreactivity, low production cost, and can be manufactured in bulk8. It is speculated that PHFs can be used as a carrier to realize the in vitro amplification of CECs and then construct engineered corneal epithelium, but there are few reports about it. The purpose of this study was to construct an engineered corneal epithelial graft in vitro with PHFs as a carrier and evaluate the feasibility of the engineered corneal epithelial graft for ocular surface reconstruction in vivo and provide experimental evidence for the treatment of ISCD in clinical practice.
1 Material and methods
1.1 Materials
1.1.1 Experimental animals Nineteen healthy and clean New Zealand white rabbits of both sexes, weighing 2-3 kg, aged 5-6months, were provided by the Animal Center of the First Affiliated Hospital of Harbin Medical University. This protocol was approved by the Ethics Committee of Scientific Research and Clinical Trials of the First Affiliated Hospital of Harbin Medical University (Approval No. 2021006). The use of laboratory animals follows the National Institutes of Health guidelines for the use and care of laboratory animals.
1.1.2 Main reagents and instruments Sebacoyl chloride (SC), glycerol ethoxylate (GE), polycaprolactone (PCL) diol (Sigma-Aldrich, USA); methyl thiazolyl tetrazolium (MTT, Sigma-Chemical, USA); TUNEL assay kit (R&D Systems, USA); sodium fluorescein (Tianjin Jingming Company); mouse anti-keratin (AE1/AE3) antibody (Beijing Zhongshan Jinqiao Company); DAPI (Santa Cruz, USA); mouse anti-p63 antibody (ab110038, Abcam, UK); goat anti-mouse IgG antibody (1583138), monkey anti-mouse IgG antibody (1608644) (LifeTechnologies, USA); tobramycin dexamethasone eye ointment (Novartis, Switzerland). Scanning electron microscope (PharosG1, Pharos, Netherlands); surgical microscope (XT-X-8A, Hunan Meixi Lake New Town Medical Investment Co., Ltd.); optical microscope (Leica DM1000, Leica, Germany); slit lamp microscope (SL-3G, Topcon, Japan).
1.2 Methods
1.2.1 Synthesis of PHFs Dihydroxy polycaprolactone (DPCL) solution was synthesized by dissolving PCL diol (0.104 g, 10 wt % PCL) in dichloromethane (15 ml). GE (0.62 ml, 0.70 mmol/L) and SC (0.30 ml, 1.42 mmol/L) were added to the DPCL solution, fully mixed, and stood at room temperature for 1 h. 7.5 ml of the solution was added to a glass culture dish with a diameter of 10 cm and placed in a vacuum oven at 60 °C for 30 min. The periphery of the membrane was removed with a sterile scalpel, and 20 ml of deionized water was added to the culture dish and soaked for 15 min. Tetrahydrofuran water was used instead of deionized water (1:1, 40 ml) to continue soaking. The film was taken out and placed in 250 ml distilled water, and the washing solution was replaced every 15 min three times. The PHFs were sterilized under 25 kGy γ irradiation.
1.2.2 Detection of physicochemical properties of PHFs (1) PHFs thickness Under low vacuum conditions, PHFs were mounted on carbon sheets, and the hydration thickness of the film was observed by scanning electron microscopy. The sample holder was used to adjust the inclination and observe the cross-section of PHFs. (2) PHFs transmittance PHFs were immersed in phosphate-buffered saline ( PBS ) for 1 h and then irradiated under ultraviolet light. The transmittance of 290-750 nm was recorded at 25°C. (3) Mechanical tensile properties of PHFs PHFs were placed in PBS solution to expand to equilibrium and cut into 2 cm × 2 cm bone-shaped films to evaluate their tensile properties. At a water bath temperature of 35°C, the clamped sample was stretched at a rate of 0.1 mm / s with a 50-N pressure measuring element until it broke in the measurement area. All calculations assume a Poisson’s ratio of 0.5.
1.2.3 Primary culture of limbal epithelial cells Four New Zealand white rabbits of clean grade were selected, and the experimental rabbits were sacrificed by anesthesia with excessive pentobarbital sodium. Eight eyeballs were quickly removed under sterile conditions, washed repeatedly with PBS and immersed in PBS containing 3 % penicillin-streptomycin double-antibody. Under the operating microscope, according to the principle of aseptic operation, the corneal tissue was washed several times with PBS containing 3 % penicillin-streptomycin double antibody, and the residual conjunctival tissue was removed. The limbal tissue including the size of the Vogt fence area about 1 mm wide was cut and moved into the ultra-clean bench. The PBS containing double antibody was washed several times again, and the tissue block with the size of about 1 mm × 1 mm × 1 mm was cut by an ophthalmologist. The tissue block was attached to the 24-well plate face-down, placed in a 37 °C, 5 % CO2 culture box for primary culture, and the medium was changed every other day. When the cells climbed to the bottom of the hole, they were subcultured, and the daily growth of the cells was observed and recorded under an inverted microscope.
1.2.4 Observation of cell adhesion under the optical microscope The cells were divided into control group and PHFs + CECs group, which were planted in ordinary 96-well plates and 96-well plates with PHFs, respectively. The cell density was 30 %. After 24 hours, the cells were observed and photographed under an ordinary optical microscope. Three fields of view with the same area were randomly selected to count the number of adherent cells, and the average number was taken.
1.2.5 Expression of keratin marker AE1/AE3 and stem cell marker p63 in cultured cells observed under a fluorescence microscope To determine the purity of primary cell culture and the dryness of corneal epithelium, AE1/AE3 and p63 staining were selected for verification. When the cells grew to about 80 %, the culture medium was discarded and washed with PBS. Fixed in 4 % paraformaldehyde for 15 min and washed with PBS. Blocked in the blocking solution (PBS+10%BSA+0.1%Triton X-100) for 1 h. Add primary antibody (AE1/AE3, p63), 4 °C wet box overnight, PBS rinse; add fluorescent secondary antibody, incubated in dark at room temperature for 1h, rinsed with PBS; DAPI was added to the nucleus for 15 min, and PBS was washed. Glycerol sealing was observed and recorded under a fluorescence microscope. The positive rate was counted according to the percentage of fluorescent staining positive cells and was calculated by the immunohistochemical HSCORE quantitative method. The experiment was repeated three times and averaged.
1.2.6 Detection of the proliferation of corneal epithelium on PHFs by MTT method The cells were divided into negative control group, positive control group and PHFs+CECs group. In the negative control group, the cells were directly planted at the bottom of the culture dish and added to the normal cell culture medium. In the positive control group, the cells were planted in the culture dish and added to the normal cell culture medium containing 100 μmol/L H2O2. In the PHF+CECs group, the cells were planted in the culture dish with PHFs and cultured in the normal cell culture medium. Each group was cultured for 24 h. Corneal epithelial cell suspensions with the same density and volume were seeded in 96-well plates, with 6 holes in each group. The 96-well plates were filled with PBS, cultured in a 5% CO2 incubator at 37 °C for 24 h, added with MTT solution 10 μl/hole, incubated in the incubator for 4 h in the dark, discarded the culture medium, added with dimethyl sulfoxide 200 μl/hole, and placed in a low-speed shaker at room temperature for 10 min. The absorbance (A490) value at the wavelength of 490 nm was measured. Each group of experiments was repeated 3 times and averaged.
1.2.7 Detection of the apoptosis of corneal epithelium on PHFs by TUNEL method Cell grouping was the same as 1.2.6. The cells were washed with PBS 3 times, fixed with 4 % paraformaldehyde for 30 min, blocked with 3% H2O2 for 10 min, penetrated with 0.1% Triton for 3 min at 4°C, incubated with TUNEL reaction mixture ( volume ratio of solution A and solution B was 1:9) for 1 h at 37°C, and stained with DAPI for 15 min. The cells were scanned and photographed under a fluorescence microscope. The TUNEL positive cell rate was calculated according to the number of TUNEL positive cells/the number of DAPI positive cells. The experiment was repeated three times and averaged.
1.2.8 Construction of LSCD rabbit model and animal experimental grouping Fifteen clean New Zealand white rabbits were used to establish the LSCD model based on the method in Reference9. The rabbits were anesthetized with pentobarbital sodium (40mg/kg) and supplemented with 2-4 mg/kg every 30 min. After anesthesia, the eyelid opener was placed, and the whole peripheral corneoscleral limbus tissue ( 2 mm inside to 2 mm outside, depth 100-150 μm ) was cut off with a micro-shear ring, and the central corneal epithelium in the range of 6 mm in diameter was scraped with a corneal curette. The successfully modeled experimental rabbits were divided into 3 groups by the random number table method, with 5 rabbits in each group, and the right eye was taken as the experimental eye. The control group was blank control, and no treatment was given after modeling. The PHFs group was the negative control. After modeling, an empty PHFs membrane with a diameter of 12 mm was placed on the corneal surface of the LSCD rabbit model. In the PHFs+CECs group, CECs were subcultured and seeded on five PHFs with a diameter of 12 mm to construct tissue-engineered grafts with a cell density of (2118±32) cells / cm2. Then the grafts were placed on the corneal surface of the LSCD rabbit model. After all the experimental animals were disposed of, a mattress-type traction suture was placed in the middle of the upper and lower eyelids, and a temporary eyelid suture was performed with a movable knot ligation. Each group was given levofloxacin eye drops and dexamethasone sodium phosphate eye drops after the operation, 3 times a day for 14 days.
1.2.9 Detection of the area of corneal defect by fluorescein sodium staining Corneal fluorescein sodium staining was performed on the day of surgery (0 d) and 3, 5, 7, 14 d after surgery. After anesthesia, the knot of the mattress suture was loosened, and 10 μl of 0.2 % sodium fluorescein solution was added to the corneal surface of experimental rabbits. After 30 s, the staining area was observed by cobalt blue light of a slit lamp microscope, and the anterior segment was photographed. Fluorescein sodium staining scoring criteria: no damage was 0 points, <1/8 damage area was 1 point, ≥1/8-<1/4 damage area was 2 points, ≥1/4-<1/2 damage area was 3 points, ≥1/2 damage area was 4 points10. The experiment was repeated three times and averaged. After the operation was completed, the mattress suture was pulled to continue to close the eyelid.
1.2.10 Detection of the histological characteristics of corneal epithelium by hematoxylin-eosin staining After 14 days, the corneas were removed for hematoxylin-eosin staining. The tissue blocks were fixed with 10% neutral formaldehyde, and dehydrated and transparent in 100%, 100%, 95%, 80% ethanol, and xylene turn. Paraffin was embedded, sliced, and then in turn in xylene, ethanol dewaxing, hydration, hematoxylin and eosin staining. Then dehydrated and transparent in different concentration gradients (100%, 100%, 95%, 80%) of ethanol and xylene, sealed with glycerin, observed and photographed under an optical microscope.
1.3 Statistical methods
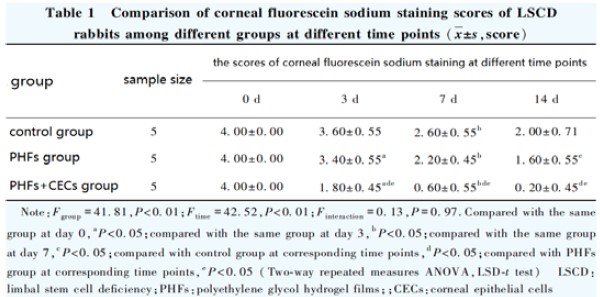
SPSS 23.0 statistical software was used for statistical analysis, and GraphPad Prism 6.0 software was used for drawing. The measurement data were confirmed to be normal distribution by the Shapiro-Wilk test and expressed as ±s. The number of adherent cells in PHFs+CECs group and control group was compared by independent sample t-test. The A490 value and TUNEL positive cell rate of PHFs+CECs group, negative control group and positive control group were compared by one-way ANOVA, and LSD-t test was used for pairwise comparison. The overall comparison of corneal fluorescein sodium staining scores at different time points in the control group, PHFs group and PHFs + CECs group was performed by two-way repeated measures ANOVA, and pairwise comparison was performed by LSD-t test. P<0.05 was considered statistically significant.
2 Results
2.1 Physicochemical properties of PHFs
PHFs is a synthetic transparent hydrogel film, which is cross-linked by DPCL, GE and SC at 60 °C (Figure 1A). Under a scanning electron microscope, the surface of PHFs is smooth, the cross section is uniform, and the thickness is not more than 150 μm (Figure 1B). The observation of UV-visible transmittance further shows that when the light moves from the UV spectrum to the visible spectrum, PHFs are similar to human corneas in increasing transmittance, with a transmittance of >99% at 400-700 nm (Figure 1C). In addition, the tensile test showed that PHFs had good tensile strength and could withstand a tensile force of about 6 MPa (Figure 1D), which could meet the requirements of surgical operation.

2.2 Expression of keratin and stem cell markers in corneal epithelium
The results of primary cell culture purity and corneal epithelial stemness showed that the cytoplasm of most cells was red after AE1/AE3 staining and the positive rate was (98.60±2.40)% (Figure 2). After p63 staining, the nuclei of most cells were green, and the positive rate was (61.20±3.60)% (Figure 3).


2.3 Comparison of cell adhesion on PHFs
The results of cell adhesion showed that the number of adherent cells in the PHFs+CECs group and the control group was (2 282.33±144.00) cells/cm2 and (2 148.00±77.60) cells/cm2, respectively, and the difference was not statistically significant (t=0.1.70, P>0.05). Continue to culture for 3 days, the cells in the two groups were a round general number (Figure 4).

2.4 Comparison of cytotoxicity of PHFs
The results of the MTT assay showed that the A490 values of PHFs + CECs group, negative control group and positive control group were 0.59±0.01, 0.65±0.07 and 0.06±0.04, respectively, and the overall difference was statistically significant (F=12.25, P<0.05). The A490 values of PHFs+CECs group and negative control group were significantly higher than those of positive control group (all P<0.05). TUNEL results showed that there were no TUNEL-positive cells in the PHFs+CECs group and the negative control group, and more TUNEL-positive cells were observed in the positive control group. The TUNEL positive cell rates of PHFs+CECs group, negative control group and positive control group were (1.68±0.25)%, (1.17±0.36)%, and (62.35±3.68)%, respectively. The overall difference was statistically significant (F=13.45, P<0.05). The TUNEL positive cell rate of PHFs+CECs group and negative control group was significantly lower than that of positive control group, and the difference was statistically significant (all P<0.05) (Figure 5).

2.5 Comparison of corneal defect area and corneal epithelial histological characteristics of LSCD experimental rabbits
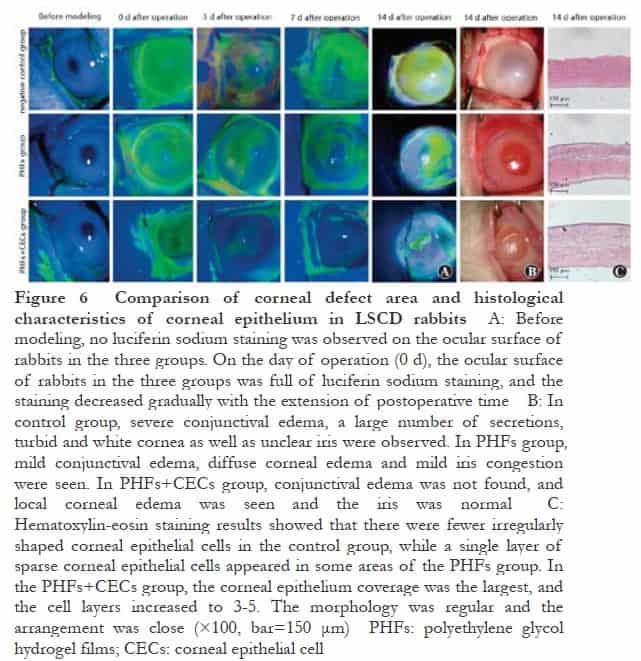
The results of fluorescein staining showed that there was no fluorescence staining on the ocular surface of the three groups before modeling. On the day of surgery (0 d), the ocular surface of the three groups of experimental rabbits was covered with green fluorescence, which proved that the LSCD model was successful. With the prolongation of postoperative time, the scores of corneal fluoresce in sodium staining in each group decreased. The scores of corneal fluorescein sodium staining in the control group, PHFs group and PHFs + CECs group decreased in turn. There were significant differences in the scores of corneal fluorescein sodium staining among the three groups at different time points (Fgroup=41.81, P<0.01; Ftime=42.52, P<0.01). The corneal fluorescein sodium staining score of the control group at 7 days after the operation was significantly lower than that at 3 days after the operation. The corneal fluorescein sodium staining scores of the PHFs group at 0, 3, 7 and 14 days after operation were significantly lower than those at the previous time point. The corneal fluorescein sodium staining scores of the PHFs+CECs group at 3 days after operation were significantly lower than those at 0 days after the operation and 7 days after operation were significantly lower than those at 3 days after the operation (all P<0.05 ). At 3, 7 and 14 days after the operation, the fluorescein sodium staining scores in the PHFs+CECs group were significantly lower than those in the control group and the PHFs group (all P<0.05 ) (Figure 6A, Table 1). At 3, 7 and 14 days after the operation, the fluorescein sodium staining scores in the PHFs+CECs group were significantly lower than those in the control group and the PHFs group (all P<0.05) (Figure 6A, Table 1). The results of hematoxylin-eosin staining showed that there were fewer irregularly shaped corneal epithelial cells in the control group, and even some areas were not re-epithelialized, which was consistent with the above fluorescein results. In the PHFs group, a single layer of sparse corneal epithelial cells appeared in some areas, and the re-epithelialization was not obvious. The coverage of corneal epithelium in PHFs+CECs group was the largest, the number of cell layers increased to 3-5 layers, the morphology was regular and the arrangement was close (Figure 6C).
3 Discussion
Tissue engineering provides a way for the mass production of corneal epithelial grafts, and overcomes the problems of limited autologous transplantation and rejection of allogeneic transplantation. However, different carriers have their advantages and disadvantages. At present, the commonly used carrier in tissue engineering is the amniotic membrane. However, as a natural biological material, amniotic membrane varies greatly among batches and has the risk of disease transmission11. The hot carriers in the field of synthetic biomaterials include collagen, silk fibroin, keratin, temperature-sensitive materials, chitosan, etc., but there are problems such as poor toughness, rapid degradation, poor transparency, and insufficient strength12. Therefore, it is imperative to find and construct a carrier scaffold that is more suitable for the growth of corneal epithelial seed cells, higher biocompatibility, and smaller immune rejection.
In this study, an ultra-thin PEG hydrogel membrane PHFs was prepared, and its feasibility as a carrier for corneal epithelial cell expansion in vitro and transplantation for the treatment of rabbit LSCD was studied at the in vitro and in vivo levels. The results showed that PHFs were thin, had strong toughness, high light transmittance, safe, non-toxic, and biocompatible. It is suitable for use as a corneal epithelial graft; pHFs can expand and transplant corneal epithelium in vitro and promote the re-epithelialization of rabbit LSCD cornea.
Hydrogel is a kind of hydrophilic polymer with a network structure, which is similar to the composition of the extracellular matrix. It can not only maintain a certain shape but also absorb a lot of water13-14. This structure makes it have good biocompatibility and high permeability to water-soluble metabolites such as glucose and oxygen15. It has been widely used in biological fields such as tissue engineering scaffolds, drug delivery systems, and soft tissue substitutes. Hydrogels can be divided into natural hydrogels and synthetic hydrogels. Natural hydrogels mainly include alginate, hyaluronic acid, chitosan, collagen, cellulose, etc.16. Synthetic hydrogels mainly include polyethylene glycol ( PEG ), polyacrylic acid, polymethyl methacrylate, polyacrylic acid-2-hydroxyethyl ester, etc.17-20. PEG hydrogel, the most important component of PHFs, has the advantages of non-toxicity, transparency, stability, good biocompatibility, low immunogenicity, inhibition of non-specific protein absorption, and fusion of biophysical and biochemical properties21-23. We previously used PHFs to amplify corneal endothelium in vitro, which provided a sufficient research basis for further amplification of corneal epithelium. In this study, through the analysis of the physical and chemical properties of PHFs, it can be seen that the synthesized PHFs have a thin thickness, smooth surface, strong toughness and high transparency, which meet the physical conditions needed to become qualified ocular surface carriers. In addition, the three chemical components used for the synthesis of PHFs, sebacoyl chloride, polyoxyethylene glyceride and polycaprolactone diol, had no chemical toxicity. Sebacoyl chloride is a diacid derived from natural sebacic acid24. Polyoxyethyl glycidyl ether is a non-toxic hydrophilic branched polymer composed of three polyethylene glycol arms. It is widely used in the textile industry, lubricating oil, metal cleaning, paper industry, printing and dyeing industry, petroleum industry and so on. Polycaprolactone diol is a biodegradable on-toxic aliphatic polyester that can impart high tensile elongation properties to materials and has been approved by the US Food and Drug Administration for human use5. The results of in vitro cytotoxicity and apoptosis experiments confirmed that PHFs and their degradation products did not affect cell proliferation.
Cell adhesion and growth of hydrogel is the key to its application in the field of tissue engineering materials. The results of the corneal limbal cell adhesion test in this study showed that the cell adhesion ability on PHFs was similar to that on ordinary Petri dishes. In previous studies using PEG as a cell carrier, due to the anti-protein properties of PEG, collagen or other adhesives must be added to the PEG material to facilitate cell adhesion and adhesion23.
In addition to the preparation of a carrier with excellent characteristics, it is also important to provide high-quality donor cells. This experiment provides corneal epithelial cells with higher stemness through the primary culture of limbal tissue. To verify the type of donor cells and calculate the proportion of stem cells, fluorescent pigments were used to observe keratin and stem cell markers. AE1 / AE3 can specifically recognize a group of acidic keratins including CK3, which is positive in the corneal epithelium at the limbus and negative in the corneal epithelium at the center of the cornea24. P63 is a nuclear transcription factor that triggers the differentiation of keratinocytes. It is mainly expressed in the basal layer of limbal epithelium in the cornea and is considered to be a specific marker of LSCs25. The donor cells obtained in this experiment are corneal epithelial cells with high purity and strong dryness, which can provide sufficient sources for the regeneration of corneal epithelium on the ocular surface of experimental rabbits in the future.
LSCs are the source of corneal epithelial cell regeneration. If the corneal stem cells are well preserved, the corneal epithelium can be completely restored within 2-3 days. Therefore, based on scraping the corneal epithelium, this study chooses to further cut off part of the limbal tissue to delay the repair of corneal epithelium, which is conducive to experimental observation. In vivo experiments, the PHFs graft carrying corneal epithelium was very beneficial to the recovery of the epithelial defect. After 14 days, the area of corneal defect was greatly reduced, and the remaining was about 5 %, which proved that the corneal epithelial cells had strong regeneration ability and could cover the entire ocular surface. In addition, corneal edema, corneal transparency and neovascularization were improved compared with the control group, which proved that PHFs were excellent carriers for carrying and transplanting corneal epithelial cells and were non-toxic to the eyes. In our previous study, PHFs corneal endothelial grafts were transplanted into the inner surface of the cornea of LSCD experimental rabbits. After 28 days of observation under a slit lamp microscope, it was also found that the cornea of the PHFs endothelial transplantation group remained transparent without corneal edema and other toxic manifestations8. Since there is no marketed and recognized ophthalmic material that can be used as a positive control in the field of synthetic biomaterials, only blank PHFs were selected as negative controls in this study. The results showed that although the PHFs graft without epithelial cells had little effect on the recovery of corneal epithelium, it could still play a certain role in repairing compared with the control group. It was speculated that the membrane reduced the damage of eyelid friction to corneal epithelial regeneration.
The results of PHFs animal experiments in this study provide a basis for further clinical trials of LSCD patients in the future. The use of PHFs will alleviate the pressure of corneal donor resources to a certain extent and broaden the idea of corneal transplantation. In addition, the synthesis of PHFs provides a new basis for corneal tissue engineering. PHFs can be used not only as a carrier for other experimental cell transplantation but also as a template for synthetic carrier materials. There are still many problems to be solved in the study of PHFs, such as whether the water content and oxygen permeability of the lens of PHFs can meet the requirements, whether the degradation products of PHFs will affect the function of other organs after entering the blood circulation, and the immune rejection of the engineered corneal epithelial graft. We will further explore this in the follow-up study.
Conflict of Interest None declared.
Author’s contribution statement Guo Yiyuan: participating in the design of experiments, implementing research, collecting and collating data, analyzing and interpreting data, and writing papers; Xian Huimin, Shereen Tan, Qiang Fu: Participate in the implementation of research, data collection, analysis and interpretation of data, thesis writing; Jinxin: Participate in design experiments, data collation; Mark Daniel, Greg G. Qiao: Participate in the analysis and interpretation of data, critical review of the knowledge content of the article; Zhang Hong: Participate in designing experiments, analyze and interpret data, and critically review the intellectual content of articles.
References
[1] Yin J, Jurkunas U.Limbal stem cell transplantation and complications[J]. Semin Ophthalmol, 2018, 33(1):134-141. DOI: 10.1080/08820538.2017.1353834.
[2] Dong Y, Peng H, Lavker RM. Emerging therapeutic strategies for limbal stem cell deficiency[J/OL]. J Ophthalmol, 2018, 2018:7894647[2021-10-09]. https://pubmed.ncbi.nlm. nih.gov/30050691/. DOI: 10.1155/2018/7894647.
[3] Guo ZH, Zhang W, Jia Y, et al. An insight into the difficulties in the discovery of specific biomarkers of limbal stem cells[J/OL]. Int J Mol Sci, 2018, 19(7):1982[2021-11-01]. https://www.ncbi. nlm.nih.gov/pmc/articles/PMC6073450/. DOI: 10.3390/ijms19071982.
[4] Zhang L, Zou D, Li S, et al. An ultra-thin amniotic membrane as a carrier in corneal epithelium tissue-engineering[J/OL]. Sci Rep, 2016, 6:21021[2021-11-01]. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4753477/. DOI: 10.1038/srep21021.
[5] Li Y, Yang Y, Yang L, et al. Poly (ethylene glycol)-modified silk fibroin membrane as a carrier for limbal epithelial stem cell transplantation in a rabbit LSCD model[J/OL]. Stem Cell Res Ther, 2017, 8(1):256[2022-01-10]. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5678789/. DOI: 10.1186/s13287-017-0707-y.
[6] Di Loreto FP, Mangione A,Palmisano E,et al. Dried human amniotic membrane as an antiadherent layer for intraperitoneal placing of polypropylene mesh in rats[J]. Surg Endosc, 2013, 27(4):1435-1440. DOI:10.1007/s00464-012-2604-x.
[7] Chen J, Yan C, Zhu M, et al. Electrospun nanofibrous SF/P(LLA-CL) membrane: a potential substratum for endothelial keratoplasty[J]. Int J Nanomedicine, 2015, 10:3337-3350. DOI: 10.2147/IJN.S77706.
[8] Ozcelik B, Brown KD, Blencowe A, et al. Biodegradable and biocompatible poly (ethylene glyeol)-based hydrogel films for the regeneration of corneal endothelium [J]. Adv Healthc Mater, 2014, 3(9):1496-1507. DO1: 10.1002/adhm.201400045.
[9] Xu B, Fan TJ, Zhao J, et al. Transplantation of tissue-engineered human corneal epithelium in limbal stem cell deficiency rabbit models[J]. Int J Ophthalmol, 2012, 5(4):424-429. DOI: 10.3980/j.issn.2222-3959.2012.04.04.
[10] Kim DW, Lee SH, Ku SK, et al. Transduced PEP-1-FK506BP ameliorates corneal injury in botulinum toxin A-induced dry eye mouse model 1[J]. BMB Rep, 2013, 46(2):124-129. DOI: 10.5483/bmbrep.2013.46.2.272.
[11] Feng Y, Borrelli M, Reichl S, et al. Review of alternative carrier materials for ocular surface reconstruction[J]. Curr Eye Res, 2014, 39(6):541-552. DOI: 10.3109/02713683.2013.853803.
[12] Zhang CW, Wu XY. Advances in the construction of tissue-engineered cornea in vitro[J].Chin JExp Ophthalmol,2017,35(2):170-174.DOI:10.3760/cma.j.issn.2095-0160.2017.02.016.
[13] Wang W, Narain R, Zeng H. Rational design of self-healing tough hydrogels: a mini review[J/OL]. Front Chem, 2018, 6:497[2022-01-10]. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6232908/. DOI: 10.3389/fchem.2018.00497.
[14] Kang-Mieler JJ, Mieler WF. Thermo-responsive hydrogels for ocular drug delivery[J]. Dev Ophthalmol, 2016, 55:104-111. DOI: 10.1159/000434694.
[15] Al-AbboodiA, Fu J, Doran PM, et al. Three-dimensional nanocharacterization of porous hydrogel with ion and electron beams[J]. Biotechnol Bioeng, 2013, 110(1):318-326. DOI: 10.1002/bit.24612.
[16] Hussain Z, Thu HE,Shuid AN,et al. Recent advances in polymer-based wound dressings for the treatment of diabetic foot ulcer: an overview of state-of-the-art[J]. Curr Drug Targets, 2018, 19(5):527-550. DOI: 10.2174/1389450118666170704132523.
[17] Amer LD, Saleh LS, Walker C, et al. Inflammation via myeloid differentiation primary response gene 88 signaling mediates the fibrotic response to implantable synthetic poly(ethylene glycol) hydrogels[J. Acta Biomater, 2019, 100:105-117. DOI: 10.1016/j. actbio.2019.09.043.
[18] Kumar D, Lyness A, Gerges I, et al. Stem cell delivery with polymer hydrogel for treatment of intervertebral disc degeneration: from 3Dculture to design of the delivery device for minimally invasive therapy [J]. Cell Transplant, 2016, 25 (12):2213-2220. DOI: 10.3727/096368916X692618.
[19] Janouková O, Pradny M, Vetrik M,et al. Biomimetic modification of dual porosity poly (2-hydroxyethyl methacrylate) hydrogel scaffolds- porosity and stem cell growth evaluation[J/OL]. Biomed Mater, 2019, 14(5):055004[2022-02-12]. https://pubmed.ncbi.nlm.nih.gov/31181551/. DOI: 10.1088/1748-605X/ab2856.
[20] Nuvoli D, Alzari V, Nuvoli L, et al. Synthesis and characterization of poly (2-hydroxyethylacrylate)/B-cyclodextrin hydrogels obtained by frontal polymerization[J]. Carbohydr Polym, 2016, 150: 166-171. DOI: 10.1016/i.carbpol.2016.05.017.
[21] Qayyum AS, Jain E, Kolar G, et al. Design of electrohydrodynamic sprayed polyethylene glycol hydrogel microspheres for cell encapsulation [J/OL]. Biofabrication, 2017, 9(2):025019[2022-01-12]. https://pubmed.ncbi.nlm.nih.gov/28516893/. DOI: 10.1088/1758-5090/aa703c.
[22] Kandile NG, Mohamed HM. Chitosan nanoparticle hydrogel based sebacoyl moiety with remarkable capability for metal ion removal from aqueous systems[J]. Int J Biol Macromol, 2019 122:578-586. DOI: 10.1016/j.ijbiomac.2018.10.198.
[23] Gong HY, Park J, Kim W, et al. A novel conductive and micropatterned PEG-based hydrogel enabling the topographical and electrical stimulation of myoblasts [J/OL]. ACS Appl Mater Interfaces, 2019, 11(51):47695-47706[2022-01-12]. https://pubmed.ncbi.nlm.nih.gov/31794187/. DOI: 10.1021/acsami.9b16005.
[24] Coutinho AB, Dd F, Souza Filho JP,et al. Cytokeratin expression in corneal dystrophies[J]. Arq Bras Oftalmol,2011,74(2):118-122. DOI: 10.1590/s0004-27492011000200010.
[25] Xu W, Liu K, Li T, et al. An in situ hydrogel based on carboxymethyl chitosan and sodium alginate dialdehyde for corneal wound healing after alkali burn[J].J Biomed Mater Res A, 2019, 107(4):742-754. DOI: 10.1002/jbm.a.36589.