Corresponding author: Jin Yuan, Email: yuanjincornea@126.com
Artificial Intelligent Ophthalmology Group, Intelligent Medicine Special Committee of China Medicine Education Association
National Key Research and Development Program of China “Development and Application of Ophthalmic Multimodal Imaging and Artificial Intelligence Diagnosis and Treatment System” Project Team
Abstract
Artificial intelligence (AI) aided diagnosis technology based on medical big data has matured in recent years. AI-aided diagnosis system based on color fundus photographs has shown favorable sensitivity and specificity in the screening of diabetic retinopathy (DR). In order to establish a unified standard for AI-assisted DR screening, promote the clinical practice of AI diagnostic system, and improve the level of DR diagnosis and treatment based on AI technology in China, the Artificial Intelligent Ophthalmology Group under the Intelligent Medicine Special Committee of China Medicine Education Association drafted and adopted the ‘Guidelines for Artificial Intelligent Diabetic Retinopathy Screening System Based on Fundus Photography’. Specifications and recommendations of AI-assisted DR diagnosis platform based on fundus photographs were formulated on system hardware parameters, equipment configuration, data collection and standards, database establishment, AI algorithm requirements, content and format of AI screening report, and AI screening follow-up plan.
Key words
Artificial intelligence; Diabetic retinopathy; Screening; Fundus photography
Guidelines register:
International Practice Guideline Registry Platform, IPGRP-2019CN038
Fund program:
National Key R&D Program (2017YFC0112400,2017YFC0112405)
Chinese versionof this article please see: Guidelines for artificial Intelligent Medicine Special Committee of China Medicine Education Association, National Key Research and Development Program of China “Development and Application of Ophthalmic Multimodal Imaging and Artificial Intelligence Diagnosis and Treatment System” Project Team. Intelligent diabetic retinopathy screening system based on fundus photography[J]. Chin J Exp Ophthalmol, 2019,37(8):593-598. DOI: 10.3760/cma.j.issn.2095-0160.2019.08.001
(中国医药教育协会智能医学专委会智能眼科学组国家重点研发计划”眼科多模态成像及人工智能诊疗系统的研发和应用”项目组. 基于眼底照相的糖尿病视网膜病变人工智能筛查系统应用指南[J].中华实验眼科杂志, 2019,37(8):593-598.DOI:10.3760/cma.j.issn.2095-0160.2019.08.001. http://zhsyykzz.yiigle.com)
1. Purpose and significance of research and application of AI-aided DR screening system
Diabetes is a common chronic disease that affects human health and quality of life. The International Diabetes Federation reports that 425 million adults suffer from various types of diabetes worldwide. China has the largest number of patients with diabetes at approximately 110 million, of which about 61 million aged 20–79 years remains undiagnosed; the undiagnosed rate is 53.6% [1]. Approximately 73.2% of patients with diabetes will manifest different types of tissue damage in the process of these patients’ survival[2]; diabetic retinopathy (DR) is a common complication of diabetes. The prevalence of DR is 18.45% in Chinese patients with diabetes [3]. Haemangioma, retinal capillary bleeding, exudation, retinal oedema, neovascularisation, fibre proliferation and tractional retinal detachment are the main pathological features of DR. DR is becoming the leading cause of blindness in adults [4].
Fundus screening can help in early diagnosis, qualitative evaluation and analysis, and timely intervention of DR. This process is of great significance to DR patients for preserving the visual function, improving the quality of life and reducing the medical burden. International, regional and local units, such as Better Diabetes Care International, Metabolic Management Centre, the Prevention and Control Centre of DR, Chinese Society of Microcirculation, National Technical Guidance Group for Preventing Blindness and Lifeline Express, have been leading a series of DR screening, prevention and treatment projects (Table 1). However, the active screening rate of DR in China remains less than 20% [5].
Table 1 DR screening in China
| Authors | Area | Screening method | Effective sample size (persons) | Diabetes patients(cases) | Prevalence rate of DR (%) |
| Jin et al[6] | Yangxi county, Guangdong province | Non-dilated fundus photography | 5 825 | 562 | 8.19* |
| Zhang et al[7] | Multicenter study | Dilated fundus photography | 15 078 | 15 078 | 27.9 |
| McGuire et al[8] | Beijing, Shantou | – | 849 | 849 | 35.2 |
| Lu et al[9] | Shanghai | Non-dilated fundus photography | 2 533 | 2 533 | 27.7 |
Note: DR: diabetic retinopathy; * : This study is about the prevalence of DR in rural population over 50 years old
The low DR screening rate is related to patients’ educational level and disease awareness, the uneven distribution of ophthalmic medical resources in China, and in particular the lack of specialised ophthalmic doctors who can undertake the screening work in primary medical institutions. At present, there are many ophthalmic image reading centres that rely on regional high-level ophthalmic medical institutions are available in China, providing technical support for the remote diagnosis and treatment of DR through the Internet and cloud transmission. However, covering an extensive range is difficult given the large workload of doctors in reading centres; moreover, the high level of working intensity leads to delayed feedback and increased misdiagnosis rates[10].
Artificial intelligence (AI) refers to the methods and technical systems created by humans to simulate and extend the characteristics of human thinking. In recent years, the AI-assisted diagnosis and treatment decision-making technology based on medical big data has become increasingly mature with the continuous improvement in the convolutional neural network, generative adversarial network , support vector machine and other AI algorithms.In particular, medical image recognition technology can perform automatic diagnosis and analysis of images in a short period by studying the existing mass imaging data, thereby making the judgment process and results more objective, accurate and efficient than those used by doctors. In 2017, a joint research team from Stanford University published an AI algorithm for skin cancer diagnosis on Nature; this algorithm has achieved an accuracy rate of more than 91%[11]. For DR screening, various AI algorithms have shown high accuracy with sensitivity and specificity exceeding 90% (Table 2) [12-15]. In April 2018, the US Food and Drug Administration approved the world’s first AI medical device, iDX-DR, for DR screening. The emergence of AI-aided diagnosis systems not only relieves the working pressure of ophthalmologists and doctors who analyse medical images, but also compensates for the uneven distribution of ophthalmological medical resources to some extent, thus exerting a profound impact on the reform of medical diagnosis and treatment modes.
Table 2 Representative research of the DR diagnosis algorithm
| Authors | Database | Sample | Sensitivity (%) | Specificity (%) | AUC | Algorithm |
| Gulshan et al.[12] | EyePACS-1
Messidor-2 |
9 963
1 748 |
90.3/97.5
87/96.1 |
98.1/93.4
98.5/93.9 |
0.991
0.990 |
Deep convolutional neural network |
| Gargeya et al.[13] | MESSIDOR 2
E-Ophtha |
75 137 | 94 | 98.0 | 0.970 | Fully data-driven artificial intelligence |
| Hemanth et al.[14] | Lotus Eye Care Hospital | 540 | 99 | 99.0 | – | Modified hopfield neural network (MHNN) |
| Li et al.[15] | Online dataset | 71 043 | 97 | 91.4 | 0.989 | Convolutional neural network |
Note:DR:diabetic retinopathy;AUC:area under the curve;-:data missing
Currently, no unified standards have been established given the diversity of data sources, algorithm models and clinical accuracy evaluation criteria of AI-aided screening systems, thus limiting the extensive application of AI-aided diagnostic systems in clinical practice[16-17]. To standardise the scientific application of AI-aided diagnosis systems in DR screening and auxiliary diagnosis and promoting the overall improvement of DR diagnosis and treatment level in China, these guidelines aim to provide recommendations for AI system users in terms of hardware equipment, data acquisition specifications, database establishment standards, algorithm evaluation and clinical application requirements and procedures of AI-aided system user firms.
2. AI Screening System Requirements for Algorithm Development and Accuracy
2.1 Source of the Training Dataset
The fundus photography (FP) images in the training dataset must come from at least two medical institutions. Sources can include online public image datasets (e.g. a dataset publicised by a scientific journal or used in an online competition), but the grading standard must comply with the methods described in Section 2.2. Any training set image not graded in accordance with the methods outlined in Section 2.2 must be regraded. Additionally, training set images must be approved by the Institutional Review Board of any relevant departments.
2.2 Grading of the Training Set Images
Each training set image must be independently graded by at least three graders, and the results must be merged by majority opinion. The graders must have a professional title in ophthalmology, have reached at least the intermediate level and acquire a relevant grading qualification.
For a single-field FP image, a grader must decide whether the image has signs of referable DR in accordance with the DR clinical diagnosis standard. For a two-field FP image pair, a grader must decide comprehensively on the basis of the two images of a single eye. If the grader decides that the image(s) shows positive signs of DR, then he/she must mark the centre coordinates of at least one sign. The grader must make his/her decision based only on the image(s) of a single eye and not on two eyes that belong to the same patient.
2.3 Quantity of the Training Set Images
A training set must contain at least 1,000 images (for the single-field FP) or 1,000 image pairs (for the two-field FP) of each positive DR stage (I, II, III and IV), as defined in the ‘Guidelines for clinical diagnosis and treatment of diabetic retinopathy in China (2014)’[19]. Furthermore, training set images must contain at least 500 images (or image pairs) of some fundus diseases in addition to DR and at least 500 images (or image pairs) of non-readable quality.
If the AI screening system lacks a designated fundus camera model, then the training set images must contain images from fundus cameras of at least five manufacturers with at least 200 images (or image pairs) per DR stage per manufacturer. The recommended demographic distributions for each DR stage are presented as follows:
- The male-to-female ratio is at or nearly 1:1.
- Images from patients older than 65 years account for a maximum of 80% of the training set.
- At least 95% of images feature patients of east Asian origin.
2.4 Standardised Testing Set
The standardised testing set must be constructed in compliance with national or industry standards. The single-field standardised testing set includes 5,000 FP images, of which 2,500 are of DR stage I and above. Similarly, the two-field standardised testing set includes 5,000 FP image pairs, wherein 2,500 pairs are of DR stage I and above. The DR negative images in the standardised testing set include 500 images (or image pairs) of other fundus diseases. When performing the test, the standardised testing set is sampled depending on whether the AI screening system has a designated fundus camera model.
2.5 Measures of the AI Algorithm/Model Performance
The discriminative performance of the algorithm in the AI screening system is measured in a single-eyed manner. If the algorithm requires single-field images, then the single-field standardised testing set is used, and each image is counted as one case; if the algorithm requires two-field images, then the two-field standardised testing set is used, and each image pair is counted as one case.
When testing, 2,000 images (or image pairs) are randomly sampled from the standardised testing set. Sampling is stratified by through the different DR stages. For the 2,000 images (or image pairs), the algorithm must provide a label that represents positive or negative which corresponds to the clinical referral standard. The algorithm must also provide a prediction value between 0 and 1 to construct the receiver operating characteristic (ROC) curve.
When testing, if the algorithm requires two-field images, then the registration and camera calibration information can be provided by the testing agency for reference.The algorithm performance measures are calculated as follows:
Sensitivity:

Specificity:

The ROC curve and area under the curve (AUC) include a series of threshold values from 0 to 1 with a step size of 0.01. The prediction values of all cases are compared with each threshold value to obtain a (sensitivity, specificity) pair for each threshold value. The ROC curve is constructed such that the horizontal axis represents 1 for specificity, whilst the vertical axis denotes sensitivity. The AUC value is calculated in accordance with the ROC curve through the trapezoid rule.
Repeatability:
Repeatability of the system on multiple images of one case. In addition to the abovementioned testing images, at least 100 cases (one eye per case) are selected. For each case, at least three FP images are applied to a single or several designated fundus camera models (Note: If the system’s algorithm is not limited to certain fundus camera models, then the sites conducting clinical trials and the testing agency must specify the appropriate models). The Fleiss’ kappa is calculated for the AI system results and serves as the measure of repeatability.
Repeatability of the system on the same image of one case. Nine groups of random transformations (random cropping with less than 5% side length of the original image, random horizontal flipping, random rotation of less than 15°) are performed on the abovementioned 2,000 images (or image pairs). The AI system must generate results on all the nine transformed image sets. The Fleiss’ kappa of the 10 groups of results (including the original image set results) is calculated as a measure of repeatability.
The algorithm performance of AI systems developed without a training set or with a small training set must be tested using a state-approved standardised testing set or a multi-centre clinical trial before application.
3. Clinical Application Standard of AI Systems
3.1 Different Types of AI Screening Systems
An offline version of the AI system can be installed on a computer or a mobile electronic device. In contrast to the online version which is required for all registering organisations, the offline system can perform clinical decision support for DR screening based on the FP image input and generate reports without connecting to the Internet. The offline version has a low configuration of hardware, does not require an Internet connection and allows for rapid response times. After loading an FP image, a screening report must be generated in less than 1 min. However, remote grading to confirm the results is infeasible with an offline system. Thus, a qualified ophthalmologist is required to be present when the system is in operation.
An optional online version of the AI system is available to companies and institutions with a certain level of Internet access ability. The online version allows users to upload FP images to the cloud for clinical decision support. Afterwards, a downloadable report is generated for confirmation. It has a high configuration of hardware and requires a fast Internet connection. The online AI system allows the confirmation of grading results through the remote grading centre in real time, thus indicating increased control and enhanced overall efficiency because an ophthalmologist is not required to be onsite. The response time for online AI systems must be less than 5 min.
3.2 Hardware Requirements of a Fundus Camera
This AI screening standard recommends a semi- or fully automatic and non-mydriatic 2D fundus camera. The specifications must comply with the following requirements:
1) angular field of view: 50° for horizontal and vertical directions when using a single view, and 45° for both directions when using two views;
2) resolving power: at least 30 lp/mm within the claimed field of view;
3) range of focus adjustment for compensation of patients’ refractive error: minimum of ±15 D
4) fixation targets: the internal fixation targets of the fundus camera must be adjustable to at least three positions, namely, optic disc centred, fovea centred and centred at the midpoint of the fovea and optic disc
5) minimum required pupil diameter for imaging: maximum of 3.3 mm
6) storage of image: the camera must support TIFF and JPG formats for lossless and lossy compressions, respectively.
3.3 Image acquisition standard
The international DR grading standard for the diagnosis and grading of DR is based on taking seven colour fundus photos with the 45° FP in accordance with the photogenic standard developed by the “Early Treatment of Diabetic Retinopathy Study” or using the ophthalmoscopy. However, these methods are unsuitable for large-scale screening given its heavy workload, high requirements on shooting technology, considerable image data size and difficulty in storage and analysis. At present, the DR screening colour fundus photos are mostly taken using a single or double field of view at the rear pole [16].
3.3.1Shooting position (1) Single field: take the midpoint of the line between the macula and optic disc as the centre of the shooting field, and the imaging must cover at least 60° of the retinal area. (2) Two-field: Field 1 takes the fovea macula as the centre of the shooting field, and the imaging covers at least 45° of the retinal area; Field 2: take the optic disc as the centre of the shooting field, and the imaging covers at least 45° of the retinal area (Figure 1).

Figure 1 Two-field method Field 1 takes the fovea of macula as the centre of the shooting field, and Field 2 takes the optic disc as the centre of the shooting field
3.3.2 Image quality requirements
Take and save at least one set of images for each eye to be used in AI analysis and doctor reading. The images should meet the following quality requirements: (1) In addition to the DR-reflected signs, such as fibre proliferation, anterior retinal haemorrhage and vitreous haemorrhage, 90% of retinal vessels in the image must be recognisable. (2) The main fundus structure is in the correct position: when using the single-field image screening, the visual field of the image must be no less than 50° in the horizontal and vertical directions. In addition, the distance from the fovea of the macula to the edge of the image must be more than 2 optic disc diameters, and the distance from the edge of the optic disc to the image is more than 2 optic disc diameters; when using the two-field image screening, the horizontal and vertical directions of each visual field must be at the minimum of 45°. The distance from the fovea of the macula to the image centre must be <1.5 optic disc diameter for fovea centred image while the distance from the centre of the optic disc to the image centre must be <1.5 optic disc diameter for optic disc centred image. The included angle between the line of optic disc and macula centre and horizontal line must not exceed 24°. (3) No shadows and/or highlighted reflective areas that can affect image interpretation within the imaging range exist. (4) The images must demonstrate moderate exposure and no overexposure and underexposures. (5) No lens stains, eyelids and/or eyelashes and/or motion artefacts must appear. (6) No other errors, such as no patient in the image or no colour fundus photos within the field, must occur.
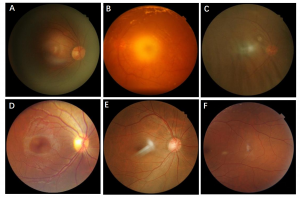
Figure 2 Common causes of poor imaging quality A: insufficient light input and underexposure of periphery B: overexposure of centre C: eyelashes shade the bottom D: poor focus due to eye movements E: lens reflections F: imaging position migration
If the image does not satisfy the abovementioned quality requirements, then the following adjustments shall be made: (1) Incorrect position of the main fundus structure: adjust the subject’s posture, adjust the fixed visual point and confirm whether the patient has strabismus or other abnormal eye conditions; then, retake the image; (2) Overexposure, underexposure and wrong focusing: adjust the exposure and focusing settings of the camera, and then retake the image; if the image is too dark, then the patient’s pupil size must be confirmed; shorten the exposure time of affected eye’s to bright light, and the brightness of the examination room must be reduced; (3) Eyelid and eyelash occlusion: remind the patient to keep his/her eyes open during the process, and assist the patient in raising his/her eyelid if necessary, and then retake the image; (4) Iris reflection: prompt the patient to focus on a fixation point, and retake the image; (5) Lens stains: check and clean the lens, and then retake the image; (6) Excessively small pupil: measure the intraocular pressure, dilate the pupil while ensuring safety and retake the image .
3.3.3 Screening report requirements
The screening report for the subject must contain the following information:(1) Basic information of the subject: name, gender, age, ID number and OD/OS; (2) Equipment information: device model, acquisition date, imaging range and image storage format; (3) AI system information: algorithm version number, algorithm applicable model and image analysis date; (4) Assessment of image quality and reliability; (5) Screening results: diagnosis of left and right eyes, the probability of disease or health, basic descriptions of the image (including but not limited to the description of haemangioma, hard exudation, soft exudation, neovascularisation, proliferating membrane and other signs) and biological measurements must be provided; (6) Description of responsibilities.
3.4 DR screening programmes
On the basis of the AI-aided system, the follow-up plan must be developed in accordance with the specific situation of high-risk patients and patients with DR. Users of the AI system are suggested to establish a verification and confirmation mechanism for the screening results of the AI system based on regional image reading centres. The screening results are only for reference before the corresponding qualified doctors confirm them, and these results have a medical effect only after the confirmation of doctors. According to the quality of patients’ images and AI results, combined with visual acuity, referral guidance is conducted for medical institutions with different levels of medical resources.
Patients with clinically diagnosed diabetes are referred to the Guidelines for Clinical Diagnosis and Treatment of DR in China (2014)[19](Table 3). The screening programme for patients with visual acuity damage and positive signs of AI screening is listed in Table 4.
Table 3 Suggestions on the first and follow-up ophthalmology examinations for different types of diabetes
| types of diabetes | the first time of ophthalmology examination | the follow-up time of ophthalmology examination |
| Type 1 | Screening can begin at age 12 for prepubescent or adolescent onset, and for postpubescent patients once diagnosed the ophthalmology examination should be taken | Once a year or as recommended by doctors |
| Type 2 | once diagnosed the ophthalmology examination should be taken | Once a year or as recommended by doctors |
| Gestational diabetes | Pre-pregnancy or first trimester | No DR, and mild, moderate NPDR:* every 3-12 months; sever NPDR: every 1-3 months |
Note: DR: diabetic retinopathy; NPDR: non-proliferative diabetic retinopathy
Table 4 Referral and follow-up recommendations for the AI screening results of DR
| visual acuity | Screening results | Follow up |
| BCVA≥0.6(or 4.8) | No obvious retinopathy, low risk, high credibility * | once a year, if dondition, the follow-up times can be increased |
| BCVA<0.6(or 4.8)or sudden loss of vision | Mild and moderate DR(stageⅠand stageⅡ),moderate to high risk,moderate to high credibility | Maintain ophthalmic follow-up, every 3 months or as recommended by doctors |
| BCVA<0.6(or 4.8)or sudden loss of vision | Sever DR(stage Ⅲand stage Ⅳ),moderate to high risk, moderate to high credibility | Ophthalmic treatment and maintain close ophthalmic follow-up, if lack of ophthalmic resources, refer to the ophthalmic diseases specialist as soon as possible |
Note: DR: diabetic retinopathy; * In accordance with the comprehensive assessment of image quality, credibility is divided into the following levels: high, medium and low credibility. When image credibility is low, the reasons must be determined, and the image must be taken again after eliminating the equipment and human interference factors. If the image remains unclear, then the patient must be referred to an ophthalmologist.
Doctors must conduct health education for all diabetic patients who have undergone screening combined with the AI results, including but not limited to the following contents: (1) Describe the contents and significance of AI-aided analysis results to patients in detail, and explain that AI-aided diagnosis is not equal to a professional physician’s diagnosis. (2) In accordance with the patient’s complaints and general condition and combined with the AI results, guide the patient to further medical resources, including but not limited to regular follow-up examinations, ophthalmic specialist visits and ophthalmic visits as soon as possible when existing high-risk factors threaten vision. (3) Inform the patients of the importance of controlling systemic conditions (blood glucose, pressure, lipids, etc.), instruct the patients to follow up the fundus regularly even if his/her vision is good or when no clear DR is present.
3.5 Data storage
Medical data refer to a detailed description of a patient’s disease and the record of treatment activities. Standardisation, normalisation and safety of data storage are crucial to preserving medical data, patient follow-up and data traceability. Data storage shall satisfy the requirements of high efficiency in planning, scientific nature of management and convenience for inquiry. The minimum details shall include the following conditions: (1) The AI system must be connected with the HIS PACS system of medical institutions to correlate the patient’s condition data. (2) The images must be stored in PNG or JPG format. (3) System analysis records and analysis results must be maintained together. (4) The offline version of the AI system must back up the data on the data storage device and update regularly, whilst the online version of the AI system can back up the data on the data storage device and update regularly.
3.6 Data safety
Medical data involves patient privacy, scientific research information and internal information of medical institutions, having its particularity and sensitivity. Extreme care must be taken in using medical data, especially when transmitting data through the Internet, to ensure the security of data and protect the relevant rights of patients. In accordance with relevant legal and regulatory requirements, such as the ‘Cyber Security Law of the People’s Republic of China’, ‘Measures for the Administration of Population Health Information’and ‘Health Insurance Portability and Accountability Act’, measurements must contain but not be limited to the following conditions: (1) Doctors and corresponding staff members at different levels must log in to the system with an account and a password and are entitled to the rights of information browsing, editing and/or data management. (2) Units and individuals must utilise and release relevant information within the scope of authorization. (3) Data storage units must be equipped with network data security protection measures. (4) Data transmitted over the Internet must be encrypted. (5) Sensitive information that do not affect the diagnosis, such as contact telephone number and home address, must be desensitised or blurred when unnecessary. (6) The landing application unit must establish a data management system, identify the responsible person and formulate an emergency plan for data leakage.
Conflict of interest:All authors declare that there is no conflict of interest
Experts group forming the guidelines:
Writing experts:
Jin Yuan , Zhongshan Ophthalmic Center, Sun Yat-sen University (Director of Artificial Intelligent Ophthalmology Group, Intelligent Medicine Special Committee of China Medicine Education Association)
Bo Lei, Department of Ophthalmology, Research Institute of Henan Province
Ming Zhang, West China Hospital Sichuan University
Yanwu Xu, Baidu Online Network Technology (Beijing) Co.,Ltd.
Haiping Ren, National Institutes for Food and Drug Control
Weihua Yang, The First People’s Hospital of Huzhou; Artificial Intelligence Key Laboratory in Medicine of Huzhou University
Xinjian Chen, Suzhou BigVisionMedical Technology Co., Ltd
Yantao Wei, Zhongshan Ophthalmic Center, Sun Yat-sen University
Peng Xiao, Zhongshan Ophthalmic Center, Sun Yat-sen University
Xiaoying Tang, Southern University of Science and Technology
Honghui Xia, Zhaoqing Gaoyao People’s Hospital
Members of Artificial Intelligent Ophthalmology Group, Intelligent Medicine Special Committee of China Medicine Education Association:
Juan Ye, The Second Affiliated Hospital of Zhejiang University School of Medicine
Di Zhao Institute of Computing Technology Chinese Academy of Science
Ruoxi Li, The 4thPeople’s Hospital of Shenyang
Yanlong Bi,Tongji Hospital of Tongji University
Weibin Chen, Guangdong EyeVision Medical Technology Co., LTD.
Wei Chen, Eye Hospital, Wenzhou Medical University
Yuzhong Chen, Beijing Airdoc Technology Co., LTD.
Yongyan Fu, The People’s Hospital of Liaoning Province
Ping Gao, Hefei Yiyun Technology Co., LTD.
Wei Han, The First Affiliated Hospital, Zhejiang University
Lingyun Hao, School of Materials Engineering, Jinling Institute of Technology
Zhiqiang He, Beijing University of Posts and Telecommunications
Jiaxu Hong, Eye and Ent Hospital of Fudan University
Jinhai Huang, Eye Hospital, Wenzhou Medical University
Bilian Ke, Shanghai General Hospital
Huiqi Li , School of Information and Electronics Beijing Institute of Technology
Qingfeng Liang, Beijing Eye Institute, Beijing Tongren Hospital, Capital Medical University
Haotian Lin, Zhongshan Ophthalmic Centre, Sun Yat-sen University
Jiang Liu, Southern University of Science and Technology
Lei Liu, The First Hospital of China Medical University
Jie Pang, 6 6 VISION TECH Co., Ltd.
Yajun Peng, Changhai Hospital, The Second Military Medical University
Hong Qi, Peking University Third Hospital
Yi Shao, The First Affiliated Hospital of Nanchang University
Hongxin Song, Beijing Tongren Hospital, Capital Medical University
Zhaoan Su, The Second Affiliated Hospital of Zhejiang University School of Medicine
Bing Wang, Baidu Online Network Technology (Beijing) Co.,Ltd.
Liqiang Wang, Chinese People’s Liberation Army General Hospital
Liangyin Wang, China Ophthalmic Nets
Yan Wang, The Affiliated Hospital of Inner Mongolia Medical University
Maonian Wu, School of Information Engineering, Huzhou University
Ling Xu , Liaoning He University
Li Yao, Beijing Chujiao Technology Co., LTD.
Lun Yu, Fuzhou University
Jianshu Yuan, Ningbo Eye Hospital
Guanghua Zhang, Shanxi Intelligence Institute of Big Data Technology and Innovation
Guisen Zhang, Inner Mongolia Chaoju Ophthalmic Hospital
Hongyan Zhang, Beijing Tongren Hospital, Capital Medical University
Hong Zhang, The First Affiliated Hospital of Harbin Medical University
Mingzhi Zhang, Joint Shantou International Eye Center of Shantou University and The Chinese University of Hong Kong
Xiulan Zhang, Zhongshan Ophthalmic Centre, Sun Yat-sen University
References
[1] International Diabetes Federation. IDF Diabetes Atlas 8th Edition[EB/OL]. (2017-12-01) [2019-03-20]. http://www.diabetesatlas.org.
[2] Investigation Group for Chronic Diabetic Complications, Chinese Diabetes Society, Chinese Medical Association. Chronic diabetic complications and related macro-vascular diseases of in-patients with diabetes in mainland of China–A national retrospective analysis in recent 10 years[J]. Chin J Diabetes, 2003, 11(4):232-237.
[3] Song P, Yu J, Chan KY, et al. Prevalence, risk factors and burden of diabetic retinopathy in China: a systematic review and meta-analysis[J]. J Glob Health, 2018, 8(1):010803. DOI: 10.7189/jogh.08.010803.
[4] Ejaz S, Chekarova I, Ejaz A, et al. Importance of pericytes and mechanisms of pericyte loss during diabetes retinopathy[J]. Diabetes Obes Metab, 2008, 10(1):53-63. DOI: 10.1111/j.1463-1326.2007.00795.x.
[5] Lu LR, Zhang LJ, Qiu ZX. Current situation for patients with diabetic retinopathy of visiting hospital and analysis on influencing factors[J]. Chin J Pract Ophthalmol, 2011, 29(11):1170-1172. DOI: 10.3760/cma.j.issn.1006-4443.2011. 11.019.
[6] Jin G, Xiao W, Ding X, et al. Prevalence of and risk factors for diabetic retinopathy in a rural Chinese population: The Yangxi Eye Study[J]. Invest Ophthalmol Vis Sci, 2018, 59(12):5067-5073. DOI: 10.1167/iovs.18-24280.
[7] Zhang G, Chen H, Chen W, et al. Prevalence and risk factors for diabetic retinopathy in China: a multi-hospital-based cross-sectional study[J]. Br J Ophthalmol, 2017, 101(12): 1591-1595. DOI: 10.1136/bjophthalmol-2017- 310316.
[8] McGuire HC, Ji L, Kissimova-Skarbek K, et al. Type 1 diabetes mellitus care and education in China: the 3C study of coverage, cost, and care in Beijing and Shantou[J]. Diabetes Res Clin Pract, 2017, 129:32-42. DOI: 10.1016/j.diabres. 2017.02.027.
[9] Lu J, Hou X, Zhang L, et al. Association between body mass index and diabetic retinopathy in Chinese patients with type 2 diabetes[J]. Acta Diabetol, 2015, 52(4):701-708. DOI: 10.1007/s00592-014-0711-y.
[10] Su BN, Li JJ, Xu L, et al. The quality evaluation of ocular images from primary hospitals in teleophthalmology[J]. Ophthalmol CHN, 2015, 24(4):230-233.
[11] Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks[J]. Nature, 2017, 542(7639):115-118. DOI: 10.1038/nature21056.
[12] Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs[J]. JAMA, 2016, 316(22):2402-2410. DOI: 10.1001/jama.2016. 17216.
[13] Gargeya R, Leng T. Automated identification of diabetic retinopathy using deep learning[J]. Ophthalmology, 2017, 124(7):962-969. DOI: 10.1016/j.ophtha. 2017.02.008.
[14] Hemanth DJ, Anitha J, Son LH, et al. Diabetic retinopathy diagnosis from retinal images using modified hopfield neural network[J]. J Med Syst, 2018, 42(12):247. DOI: 10.1007/s10916-018-1111-6.
[15] Li Z, Keel S, Liu C, et al. An automated grading system for detection of vision-threatening referable diabetic retinopathy on the basis of color fundus photographs[J]. Diabetes Care, 2018, 41(12):2509-2516. DOI: 10.2337/dc18- 0147.
[16]林浩添, 吴晓航. 加快基于眼科图像数据库的眼病人工智能辅助诊断平台建设[J] . 中华实验眼科杂志,2018,36( 8 ): 577-580. DOI: 10.3760/cma.j.issn.2095-0160.2018.08.001
Lin HT, Wu XH. Accelerating the construction of artificial intelligence diagnostic platform based on ophthalmic imaging database[J]. Chin J Exp Ophthalmol, 2018, 36(8):577-580. DOI: 10.3760/cma.j.issn.2095-0160.2018.08.001.
[17]张秀兰, 李飞. 人工智能和青光眼:机遇与挑战[J] . 中华实验眼科杂志,2018,36(4): 245-247. DOI: 10.3760/cma.j.issn.2095-0160.2018.04.002
Zhang XL, Li F. Artificial intelligence and glaucoma:opportunities and challenges[J]. Chin J Exp Ophthalmol, 2018, 36(4):245-247. DOI: 10.3760/cma.j. issn.2095-0160.2018.04.002.
[18] Govinda A, de Verteuil R. Systematic review of the diagnostic accuracy of the single, two and three field digital retinal photography for screening diabetic retinopathy[J]. JBI Libr Syst Rev, 2011, 9(16):491-537.
[19]Guidelines for clinical diagnosis and treatment of diabetic retinopathy in China (2014)[J]. Chin J Ophthalmol, 2014, 50(11):851-865. DOI: 10.3760/cma.j.issn. 0412-4081.2014.11.014.