·Clinical Research·
Evaluation of the efficacy of rAAV2-ND4 intravitreal injection for Leber’s hereditary optic neuropathy
Li Xin1, Tian Zhen1, Chen Zhang 1, Li Bin1 ,2 ,3, Zhang Yong 1
1Department of Ophthalmology, Shiyan Taihe Hospital, Affiliated Hospital of Hubei University of Medicine, Shiyan 442000, China; 2Department of Ophthalmology, Tongji Hospital, Tongji Medical College of Huazhong University of Science and Technology, Wuhan 430030, China; 3Neurophth, Wuhan 420200, China
Corresponding author: Yong Zhang, Email: inforzy@163.com
[Abstract] [View PDF in English] [View PDF in Chinese] [Read Full Text]
Objective To evaluate the safety and clinical outcomes of gene therapy for Leber’s hereditary optic neuropathy (LHON).
Methods A multi-center, prospective, non-randomized, controlled clinical trial was designed and 40 patients with mitochondrial DNA 11778 mutation in 80 eyes were recruited between December 2017 and February 2018 at the Department of Ophthalmology, Shiyan Taihe Hospital. Recombinant adeno-associated virus 2-complex I NADH dehydrogenase 4 (rAAV2-ND4) was intravitreally injected into the eye with poor visual acuity (or the right eye when both eyes had equal visual acuity). The contralateral eye was designated as the uninjected eye, thus forming an injected eye group and an uninjected eye group, with 40 eyes in both groups. Best-corrected visual acuity (BCVA) was determined using a tumbling E logarithmic visual acuity chart before surgery and 1, 3, 6, and 12 months after surgery. Intraocular pressure (IOP) was determined using a non-contact tonometer. The anterior segment of the eye was observed using a slit lamp microscope. The fundus was examined using a CR-2 non-mydriatic retinal camera. Visual acuity, changes in IOP, and complications were compared between the injected and uninjected eye groups before and after gene therapy. The efficacy of the treatment was evaluated, and an improvement in BCVA by teriorlogMAR was considered effective.
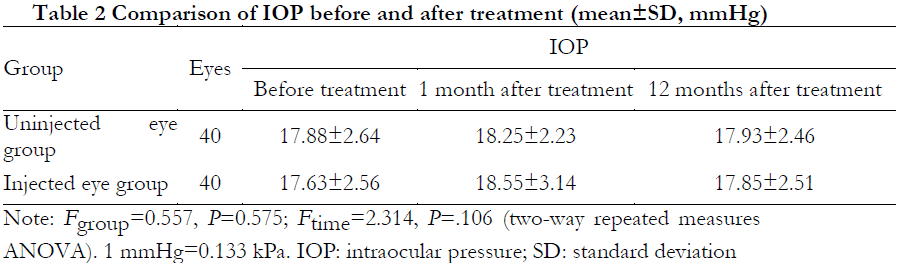
Results Among the 40 patients, visual acuity was improved by slit logMAR in 23 patients for an efficacy of 57.5%, including six eyes in the injected eye group, four eyes in the uninjected eye group, and both eyes in 13 cases. BCVA at 12 months after treatment was (1.51 ± 0.62) logMAR in the uninjected eye group and (1.62 ± 0.58) logMAR in the injected eye group, which were significantly improved from the pre-treatment BCVA of (1.75 ± 0.46) logMAR and (1.83 ± 0.47) logMAR, respectively (P<0.01 for both groups). The overall difference in BCVA between the two groups was not statistically significant (Fgroup=0.084, P=0.772). The overall difference in IOP before and after treatment was not statistically significant (Fgroup=0.557, P=0.575; Ftime=2.314, P=0.106). No serious complications occurred in any patient after treatment or during the follow-up period.
Conclusions The rAAV2-ND4 intravitreal injection for LHON was safe and effective, and monocular intravitreal injection of the therapeutic gene could improve visual acuity in both eyes.
[Key words] Leber’s hereditary optic neuropathy; recombinant adeno-associated virus 2-complex I NADH dehydrogenase 4; gene therapy; visual acuity; intravitreal injection
Funding program: Shiyan Taihe Hospital Funding Projects (2019JJXM004, 2020JJXM022)
Clinical trial registration no.: Clinical Trials.gov, NCT03153293
DOI: 10.3760/cma.j.cn115989-20210330-00218.
Leber’s hereditary optic neuropathy (LHON) is a hereditary eye disease caused by mutations in mitochondrial DNA (mtDNA), often at position 11778. [1] It manifests as simultaneous or sequential acute or subacute loss of visual acuity in both eyes. [2] It tends to develop in adolescent males and is a common cause of bilateral blindness in this population. Many patients experience a dramatic reduction in visual acuity to < 0.1 in both eyes within a relatively short period of time. Current treatments for LHON include oral Q10, idebenone, and EPI-743, [3-5] but none of these treatments have been shown to be effective. We previously conducted a series of basic studies to confirm that recombinant adeno-associated virus 2-complex I NADH dehydrogenase 4 (rAAV2-ND4) could be normally expressed in the mitochondria or enter the mitochondria of cells after injection, [6] thus validating the safety and efficacy of intravitreal rAAV2-ND4 injection and determining the dosage. [7] After completing this preliminary basic research, we conducted the first clinical gene therapy trial in nine patients with LHON in 2011–2012 and found that six patients had significantly improved visual acuity with no serious complications after a follow-up for 36 months, [8-9] thereby initially confirming the safety and efficacy of gene therapy for LHON. Since then, research groups from France and the United States have conducted clinical trials of gene therapy for LHON without serious ocular complications. [10-11] To further expand the sample size to confirm the safety and efficacy of gene therapy, we conducted a second clinical trial of gene therapy for LHON to provide a foundation for evaluating the methodology and clinical efficacy of rAAV2-ND4 intravitreal injection for the treatment of LHON.
1 Materials and methods
1.1 General characteristics
The study was designed as a multi-center, prospective, nonrandomized, controlled clinical trial. The research group consisted of clinicians from Shiyan Taihe Hospital, Tongji Hospital of Tongji Medical College of Huazhong University of Science and Technology, Ezhou Central Hospital of Wuhan University, and Hospital Oftalmológico Dr. Pedro Lagleyze, Buenos Aires, Argentina. Forty patients with LHON caused by mutations in mtDNA position 11778 were recruited between December 2017 and February 2018 at the Department of Ophthalmology of Shiyan Taihe Hospital. The eye with the poorer visual acuity (or the right eye when both eyes had equal visual acuity) was selected for rAAV2-ND4 intravitreal injection as the injected eye group and the contralateral eye was selected as the uninjected eye group, with 40 eyes in both groups. The LHON diagnostic criteria were: (1) painless vision loss with acute or subacute onset; (2) monocular or binocular onset; (3) prolonged P100 wave latency and reduced amplitude as indicated by visual evoked potentials; (4) central visual field loss or color vision deficiency; (5) normal or mildly hyperemic or edematous optic disc at early stages, localized microvascular dilatation without hemorrhage or exudation in the peripapillary region, temporal pallor of the optic disc at late stages; (6) gene sequencing results consistent with a diagnosis of LHON. The inclusion criteria were: (1) diagnostic criteria of LHON met, confirmed as a mutation at mtDNA position 11778 by gene sequencing, no other treatment in the past 3 months, and no improvement in visual acuity; (2) age 12–65 years, disease duration of 36 months, and stable visual acuity; (3) agreement not to use other drugs or treatments for this disease during the treatment period. The exclusion criteria were: (1) chronic diseases such as diabetes mellitus, severe cardiovascular, cerebrovascular, hepatic, renal, or other primary disease, other systemic neurological diseases, currently recovering from heart surgery, psychiatric disorders, or cancer; (2) other eye diseases affecting vision such as glaucoma; (3) participation in other clinical studies within the previous 3 months; (4) a history of alcohol, tobacco, drug, or substance abuse or exposure to toxic substances; (5) pregnancy, lactation, or planning to become pregnant within 12 months; (6) significant abnormalities in preoperative immunological tests, such as positive humoral immune response to AAV2 or CD3/CD8 abnormalities. All 40 patients had binocular eye disease, of whom 33 patients were male and 7 were female. The disease duration ranged from 7 to 312 months with a mean duration of (41.30 ± 56.08) months. The patients were aged from 12–42 years with a mean age of (20.30 ± 6.39) years. The mean best-corrected visual acuity (BCVA) in the injected and uninjected eyes was (1.83 ± 0.47) logMAR and (1.75 ± 0.46) logMAR, respectively, which were not statistically significantly different (t = 0.813, P=0.419). The mean baseline intraocular pressure (IOP) in the injected and uninjected eyes was (17.63 ± 2.56) mmHg and (17.88 ± 2.64) mmHg, respectively (1 mmHg = 0.133 kPa), which were not statistically significantly different (t = 0.430, P=0.669). The trial was conducted in accordance with the Declaration of Helsinki, and the study protocol was approved by the Institutional Review Board of Shiyan Taihe Hospital (approval number: 201807). All patients and/or guardians voluntarily provided informed written consent after being fully informed of the trial content and surgical risks.
1.2 Methods
1.2.1 Surgical procedure The operation was performed in the operating room with the patient in the supine position. The eye was disinfected with three drops of propantheline chloride ophthalmic solution. The face and eye were routinely disinfected and draped. The eye was opened with an eye speculum, the conjunctival sac was flushed with 0.5% v/v povidone iodine disinfectant and washed with 0.9% w/v sodium chloride solution after 10 s to remove the disinfectant. A 1-mL syringe was used to aspirate 0.05 mL of rAAV-ND4 (1 × 1010 vg) (Neurophth, Wuhan, China), which was injected 4 mm behind the corneal margin at a 7–8 o’clock position clockwise along the eye. The drug was slowly injected, the needle was withdrawn, and a sterile cotton swab was pressed against the injection site for 2 min. Antibiotic ointment was applied to the operated eye, which was covered, and the patient was returned to the ward after lying in the supine position for 30 min. All operations were performed by the same senior physician.
1.2.2 Follow-up and evaluation indicators Visual acuity, IOP, and the anterior segment and fundus of the eye were examined regularly at 1, 3, 6, and 12 months after surgery. Therapeutic efficacy was defined as an improvement in visual acuity by ≥ 0.3 logMAR at the time of the final follow-up. The primary endpoint was the change in visual acuity, which was determined by the same physician using a tumbling E logarithmic visual acuity chart (Wenzhou Xingkang Medical Tech. Co., Ltd., Wenzhou, China) and converted into logMAR visual acuity. IOP was measured three times using an air pulse non-contact full auto tonometer (Canon, Tokyo, Japan), and the mean value was used. The anterior segment of the eye was examined using a slit lamp microscope, and the fundus was examined using a CR-2 non-mydriatic retinal camera (Canon).
1.3 Statistics
SPSS 22.0 software was used for statistical analysis. The Shapiro-Wilk test was applied to determine the normality of measurement data, and the data are presented as mean ± standard deviation. Count data are presented as frequency and percentage. The overall comparison of BCVA and IOP before and after gene therapy in the injected and uninjected eye groups was analyzed by two-way repeated measures analysis of variance ANOVA, and the least significant difference-t test was used for multiple comparisons. Differences with P<0.05 were considered to be statistically significant.
2 Results
2.1 Therapeutic efficacy
Following treatment, the visual acuity was improved in six eyes in the injected eye group and in four eyes in the uninjected eye group, as well as in 13 cases in both eyes. The visual acuity of 23 of the 40 patients was improved by ≥ 0.3 logMAR, for an efficacy of 57.5%.
2.2 Comparison of BCVA before and after treatment in both groups
The difference in BCVA between the two groups was not statistically significant (Fgroup=0.084, P=0.772), whereas the difference in BCVA before and after treatment was statistically significant (Ftime=34.109, P<0.01). At 12 months after treatment, BCVA was statistically significantly improved in both groups compared to before treatment (both P<0.01) (Table 1).
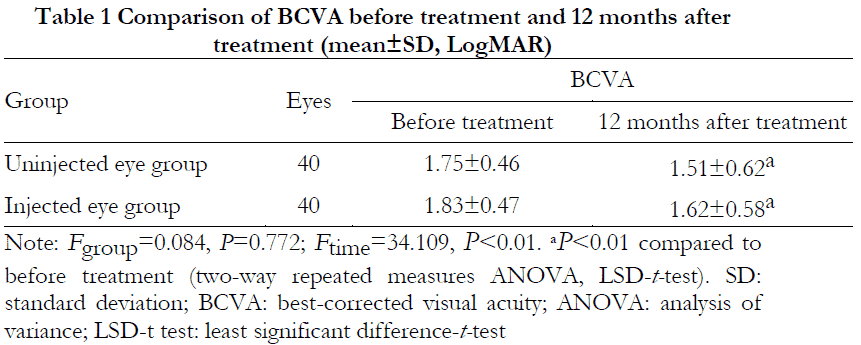
2.3 Comparison of IOP before and after treatment in both groups
The overall difference in IOP between the two groups before and after treatment was not statistically significant (Fgroup=0.557, P=0.575; Ftime=2.314, P=0.106) (Table 2).
2.4 Complications
During the follow-up period, there were no cases of conjunctivitis, keratitis, anterior uveitis, intermediate uveitis, or retinal detachment in any of the patients after rAAV2-ND4 intravitreal injection. In two eyes with high IOP (approximately 26 mmHg) after injection, IOP was returned to normal after treatment with carteolol HCl ophthalmic solution b.i.d.
3 Discussion
In 2007, three research groups in the United States and the United Kingdom successfully conducted a clinical trial on gene therapy for Leber’s congenital amaurosis, [12-13] ushering in the era of gene therapy for eye diseases. LHON is caused by a mutation at mtDNA position 11778 that changes the amino acid at position 340 of the ND4 gene in the respiratory chain from arginine to histidine, resulting in dysfunction of the ND4 protein. Gene therapy involves the transfer of the non-mutated ND4 gene into the patient’s cells at the site of the disease to encode a normal ND4 protein, replacing the function of the mutated ND4 protein. Gene therapy is currently the most promising treatment for LHON, but its safety is the primary issue that must be addressed. Because intravitreal injection of AAV2 vectors can induce immune rejection in the body and thus affect efficacy, we determined the timing and peak of the immune response induced by AAV2 and the drug regimen in preliminary animal experiments, [14] thus reducing the risk for immune rejection in the body. Gensight Biologics (Paris, France) and Guy et al. (USA) used AAV2 as a vector to treat LHON patients with varying degrees of intermediate or anterior uveitis. [10-11] Subsequently, Gensight Biologics confirmed an association of intraocular inflammation with the AAV2 vector and suggested that prophylactic systemic administration of glucocorticoids could reduce the inflammatory response. [15] In the present study, glucocorticoids were administered to all patients 1 week before and 4 weeks after gene therapy to reduce the probability of immune rejection, and no serious complications occurred during the intraoperative or postoperative follow-up periods, confirming the safety of the gene therapy.
Between 2011 and 2012, we treated nine patients with LHON with rAAV2-ND4 for the first time and conducted a follow-up on the patients for 7 years. Six patients exhibited significant improvement in visual acuity (≥ 0.3 logMAR), for an efficacy of over 60%. [16] The results of the present study showed that the efficacy of rAAV2.ND4 intravitreal injection was 57.5%, consistent with the results of the previous study and further confirming the efficacy of LHON gene therapy. In addition, it is of note that the results of both gene therapy treatments suggested that not only did the visual acuity of the injected eye improve compared to the baseline, but the visual acuity of the uninjected eye also improved to various degrees. Man et al. [17] selected one eye in 37 LHON patients with the m.11778C mutation for rAAV2/2.ND4 treatment by random assignment, and observed sustained improvement in binocular visual acuity over a follow-up period of 96 weeks. The results showed that at week 96, 68% (25/37) of the subjects had clinically relevant recovery of BCVA from baseline levels in at least one eye, and 78% (29/37) had improved binocular vision, indicating that monocular gene therapy can significantly improve binocular vision. The mechanisms responsible for this binocular vision improvement remain poorly understood. In a non-human primate study, viral vector DNA was found to be transferred from the injected eye to the anterior segment, retina, and the optic nerve of the contralateral uninjected eye (https:/www.gensight-biologics.com). In addition, Yang et al. [18] performed intravitreal injection of fluorescent gold in one eye of rats, and after 2 weeks, the fluorescent gold was found to travel up to the contralateral optic chiasma, after which it partially entered the optic nerve of the contralateral eye. This provides a plausible explanation for the improved binocular visual acuity after monocular gene therapy injection, but whether the substance is transferred via axonal transport, axonal regeneration, or other means remains unclear and needs to be further investigated.
The importance of the present study is that there have been no reports on the clinical practice of gene therapy in China, only references and summaries of foreign literature. Additionally, this study provided a clinical foundation for gene therapy of LHON in China. Because a normal control group was not established in the present study, and that the gene therapy in one eye changed the visual function of both eyes, the eyes that did not undergo intravitreal injection of rAAV2.ND4 cannot be used as a control group. Therefore, the changes in visual acuity before and after gene therapy were compared in 80 eyes of 40 patients. A limitation of the present study was that the natural course of the disease was not set as the control group, and an analysis of patients with the natural course of the disease was not available. The present study did not analyze the correlation factors between patients with and without improvement of visual acuity, and did not include objective tests such as visual field testing, retinal nerve fiber layer thickness measurements, or visually evoked potential measurements.
The results of the present study showed that rAAV2-ND4 intravitreal injection was safe and effective in the treatment of LHON, thereby providing a theoretical foundation for future development of clinical gene therapies for LHON. We will continue to perform follow-up on the 40 patients over an extended period of time, observe their general condition, and evaluate the improvement in their visual function after gene therapy with objective examinations, including visual field testing, retinal nerve fiber layer thickness measurements, and visually evoked potential measurements to investigate the long-term safety and efficacy of gene therapy for LHON.
Conflict of interest: B.L. is the Chairman of the Board of Neurophth, Wuhan, China and the developer of the gene therapy drug rAAV2/2-ND4. The gene therapy drug used in this trial was produced by Neurophth, Wuhan, China. The trial was conducted at a public first-class tertiary hospital. All data were obtained by objective examination and the results of the trial were not affected. All remaining authors declare no conflict of interest.
References
[1] Mackey DA, Oostra RJ, Rosenberg T, et al. Primary pathogenic mtDNA mutations in multigeneration pedigrees with Leber hereditary optic neuropathy[J]. Am J Hum Genet, 1996, 59(2):481-485.
[2] Yang M, Wei SH, Xu QG. Clinical features of Leber hereditary optic neuropathy[J]. Ophthalmol CHN, 2017, 26(5):343-346. DOI: 10.13281/j.cnki. issn.1004-4469.2017.05.012.
[3] Mashima Y, Kigasawa K, Wakakura M, et al. Do idebenone and vitamin therapy shorten the time to achieve visual recovery in Leber hereditary optic neuropathy?[J]. J Neuroophthalmol, 2000, 20(3):166-170. DOI: 10.1097/ 00041327-200020030-00006.
[4] Rudolph G, Dimitriadis K, Büchner B, et al. Effects of idebenone on color vision in patients with leber hereditary optic neuropathy[J]. J Neuroophthalmol, 2013, 33(1):30-36. DOI: 10.1097/WNO.0b013e318272c643.
[5] Sadun AA, Chicani CF, Ross-Cisneros FN, et al. Effect of EPI-743 on the clinical course of the mitochondrial disease Leber hereditary optic neuropathy[J]. Arch Neurol, 2012, 69(3):331-338. DOI: 10.1001/archneurol.2011.2972.
[6] Yang S, Liu L, Pei H, et al. Study on transfection of adeno associated virus 2-ND4 gene into mitochondria[J]. Chin J Exp Ophthalmol, 2014, 32(8):693-695. DOI: 10.3760/cma.j.issn.2095-0160.2014.08.15.
[7] Shi H, Gao J, Pei H, et al. Adeno-associated virus-mediated gene delivery of the human ND4 complex I subunit in rabbit eyes[J]. Clin Exp Ophthalmol, 2012, 40(9):888-894. DOI: 10.1111/j.1442-9071.2012.02815.x.
[8] Wan X, Pei H, Zhao MJ, et al. Efficacy and safety of rAAV2-ND4 treatment for Leber’s hereditary optic neuropathy[J/OL]. Sci Rep, 2016, 6:21587[2021-03-10]. https://pubmed.ncbi.nlm.nih.gov/26892229/. DOI: 10.1038/srep21587.
[9] Yang S, Ma SQ, Wan X, et al. Long-term outcomes of gene therapy for the treatment of Leber’s hereditary optic neuropathy[J]. EBioMedicine, 2016, 10:258-268. DOI: 10.1016/j.ebiom.2016.07.002.
[10] Vignal C, Uretsky S, Fitoussi S, et al. Safety of rAAV2/2-ND4 gene therapy for Leber hereditary optic neuropathy[J]. Ophthalmology, 2018, 125(6):945-947. DOI: 10.1016/j.ophtha.2017.12.036.
[11] Guy J, Feuer WJ, Davis JL, et al. Gene therapy for Leber hereditary optic neuropathy: low- and medium-dose visual results[J]. Ophthalmology, 2017, 124(11): 1621-1634. DOI: 10.1016/j.ophtha.2017.05.016.
[12] Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis[J]. N Engl J Med, 2008, 358(21):2240-2248. DOI: 10.1056/NEJMoa0802315.
[13] Bainbridge JW, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis[J]. N Engl J Med, 2008, 358(21):2231-2239. DOI: 10.1056/NEJMoa0802268.
[14] Gao J, Shi H, Pei H, et al. Comparison of immunosuppressive effects and ND4 Expression among Different Immunosuppressive Strategies following AAV2-ND4 gene treatment for leber hereditary optic neuropathy[J]. Acta Med Univ Sci Technol Huazhong, 2013, 42(2):187-191. DOI: 10.3870/j.issn.1672-0741.2013.02.013.
[15] Bouquet C, Vignal Clermont C, Galy A, et al. Immune response and intraocular inflammation in patients with Leber hereditary optic neuropathy treated with intravitreal injection of recombinant adeno-associated virus 2 carrying the ND4 gene: a secondary analysis of a phase 1/2 clinical trial[J]. JAMA Ophthalmol, 2019, 137(4):399-406. DOI: 10.1001/jamaophthalmol.2018.6902.
[16] Yuan J, Zhang Y, Liu H, et al. Seven-year follow-up of gene therapy for Leber’s hereditary optic neuropathy[J]. Ophthalmology, 2020, 127(8):1125-1127. DOI: 10.1016/j.ophtha.2020.02.023.
[17] Yu-Wai-Man P, Newman NJ, Carelli V, et al. Bilateral visual improvement with unilateral gene therapy injection for Leber hereditary optic neuropathy[J/OL]. Sci Transl Med, 2020, 12(573):eaaz7423[2021-03-15]. https://pubmed.ncbi.nlm. nih.gov/33298565/. DOI: 10.1126/scitranslmed.aaz7423.
[18] Yang S, He H, Zhu Y, et al. Chemical and material communication between the optic nerves in rats[J]. Clin Exp Ophthalmol, 2015, 43(8):742-748. DOI: 10.1111/ceo.12547.