·Clinical Research·
Comparison of the clinical outcomes of ophthalmic artery chemotherapy with systemic vein-eye artery chemotherapy for retinoblastoma in children
Di Qi1, Shen Gang2, Shi Shengli1, Lu Yuebing1, Zhang Jing3, Liu Jing1, Hu Jing1, Chen Zhiping1
1Department of Radiology, Chidren’s Hospital Affiliated of Zhengzhou University, Henan Children’s Hospital, Zhengzhou 450018, China; 2Interventional Hemangioma Department, Children’s Hospital of Capital Institute of Pediatrics, Beijing 100020, China; 3Department of Hemangioma Intervention, Guangzhou Women and Children’s Medical Center, Guangzhou 510623,China
Di Qi is now working at the Interventional Hemangioma Department, Children’s Hospital of Capital Institute of Pediatrics, Beijing 100020, China
Corresponding author: Chen Zhiping, Email: workerchen123@163.com
[Abstract] [View PDF in English] [View PDF in Chinese] [Read Full Text]
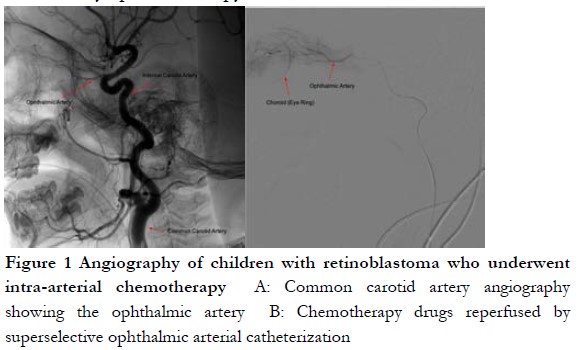
Objective This study aimed to compare and analyze the clinical efficacy, prognosis, and complications of intravenous chemotherapy (IVC) combined with intra-arterial chemotherapy (IAC) and only IAC retinoblastoma (RB) of children. Methods: A cohort study was conducted to follow up 300 children treated by intraocular RB in Children’s Hospital Affiliated to Zhengzhou University from June 2015 to June 2019. The children were divided into two groups based on different treatment methods: IAC combined with local laser photocoagulation or cryotherapy was performed in 160 eyes of 140 cases as IAC group, and IVC combined with IAC treatment was employed in 192 eyes of 160 cases as IVC + IAC group. All children were followed up for 2–60 months. The eye-saving rate, survival rate of the patients, and treatment-related complications were compared between two groups.
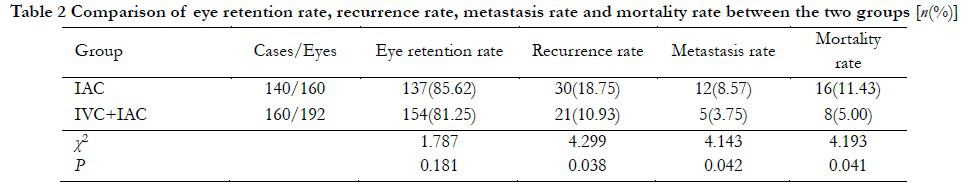
Results The eye-saving rate was 85.62% (160 eyes) in the IAC group and 81.25% (192 eyes) in the IVC + IAC groups, respectively, and no statistical difference was observed between the two groups (P>0.05). The recurrence rate and metastasis rate in IAC group were 18.75% and 8.57%, respectively, which were significantly higher than 10.93% and 3.75% of IVC + IAC group (χ2=4.299, P=0.038 and χ2=4.143, P=0.041, respectively). No significant difference was found in 1-year survival rate between the two groups (χ2=1.766, P=0.184). The overall survival rate in IVC + IAC group was 95.00%, which was significantly higher than 88.57% in IAC group (χ2=4.193, P=0.041). Kaplan-Meier analysis showed that the overall recurrence, metastasis, and overall survival rates in IVC + IAC group were better than those of the IAC group (all P<0.05). Regarding to the treatment-related complications, the incidences of eyelid edema, ptosis, fundus hemorrhage, enophthalmos, and cataract in IAC group were not significantly different from the IVC + IAC group (all at P>0.05). The incidence of myelosuppression in IAC group was 32.14%, which was significantly lower than 42.75% in IVC + IAC group (χ2=4.255, P=0.039).
Conclusions Compared with only IAC treatment, IVC combined with IAC for RB of children reduce the metastasis and recurrence of
RB and improved survival, but IVC + IAC therapy increase the incidence of systemic complications.
[Key words] Retinoblastoma; Chemotherapy; Ophthalmic artery perfusion; Survival rate; Recurrence rate; Complications
Fund program: Henan Medical Science and Technology Research Program Joint Construction Project (LHGJ20190950); Natural Science Foundation of Guangdong Province (2015A030313878)
DOI: 10.3760/cma.j.cn115989-20200917-00652
Retinoblastoma (RB) is a common primary intraocular malignant tumor in infants, accounting for about 3% of childhood cancers 1. Most RBs occur before 3 years of age, with incidences of 1/18 000 -1/16 000, indicating that RB is a serious threat to the vision and life of children 2. With the continuous improvement of RB diagnosis technology and the update of treating concepts, the treatment principle of RB has changed from saving children’s lives to saving children’s lives while preserving eyeballs and vision to improve the quality of life 3. Clinical studies showed that intravenous chemotherapy (IVC) combined with local laser photocoagulation or cryotherapy significantly improved the survival and eye preservation of patients with RB 4, but had less eyeball preservation for advanced RB, and sometimes caused serious complications, such as myelosuppression and hearing loss, thereby limiting its clinical application 5. Intra-arterial chemotherapy (IAC) is a treating method of injecting chemicals into the ophthalmic artery through the catheter to increase the local drug concentration of eye tumors and improve the eye preservation during intraocular RB 6. However, IAC sometimes causes ocular vascular embolism, and local chemotherapy may not be able to control potential metastatic tumor cells 5. Some studies showed that IVC combined with IAC in the treatment of advanced intraocular RB improved eye preservation and reduced the risk of complications and metastasis 7. At present, the study of IVC combined with IAC in the treatment of RB is predominantly focused on the efficacy of IAC after the failure of IVC, but studies on the clinical efficacy of IVC combined with IAC and IAC alone in the treatment of RB are minimal. In this study, patients with RB treated by different methods were followed up to compare the efficacy, prognosis, and adverse reactions of IAC and IVC combined with IAC in the treatment of RB, as well as to provide a reference for clinical treatments of RB.
1 Materials and methods
1.1 General information
A cohort study was conducted to follow up 300 children with intraocular RB who were hospitalized in Henan Children’s Hospital (Zhengzhou, China) from June 2015 to June 2019. The children aged 6–77 months, including 180 male and 120 females. Fifty-two patients were binocular RB and 248 patients were monocular diseases.
The inclusion criteria were as follows: (1) children who were clinically diagnosed with intraocular stage RB and had affected eyes belonging to stages B, C, D, and E, confirmed in accordance with the International Intraocular Retinoblastoma Classification 8; (2) new cases with no relevant treatment received before the test treatment; and (3) complete clinical and follow-up data. The exclusion criteria were as follows: children with (1) tumors invading the optic nerve and causing intracranial or systemic metastasis; (2) children suffering from cataract, neovascular glaucoma, iris neovascularization, or other serious eye diseases; (3) children suffering from serious systemic diseases, such as in liver, kidney, and blood; or (4) children who failed to continue treatment due to serious adverse events. In accordance with different treatment methods, children were divided into two groups: IAC (140 cases, 160 eyes; receiving IAC combined with local laser photocoagulation or cryotherapy) and IVC + IAC (160 cases, 192 eyes; treated with IVC twice before IAC combined with local therapy). No significant difference in general data was observed between the two groups (P>0.05, Table 1). This study was approved by the Ethics Committee of Children’s Hospital Affiliated to Zhengzhou University (approval number: 20150503), and all family members/guardians of the patients understood the purpose and method of this study, and voluntarily signed the informed consent form.
1.2 Methods
1.2.1 Treatment Plan (1) IAC combined with local treatment: the interval of each treatment was 3–4 weeks, and the specific number of treatments was determined by the results of fundus examinations after each IAC. Different methods of administration were adopted: the first infusion chemotherapy with melphalan (H20110; Excella, Feucht, Germany) combined with carboplatin (H10920028, 20 mg; Qilu Pharmaceutical, Jinan, China); second time: melphalan combined with topotecan (H20070204, 0.5–1.0 mg; Nanjing Ruinian Best Pharmaceutical, Nanjing, China) infusion chemotherapy; third time: melphalan combined with carboplatin (20 mg) infusion chemotherapy; fourth time: melphalan combined with topotecan (0.5–1.0 mg), and the combination was used alternately. The dose of melphalan for the first time was determined in accordance with the age and body weight of the children, and the dose was adjusted appropriately in accordance with the reaction and tumor changes in the children after the previous IAC 9. (2) Combined IVC + IAC treatment: IVC was performed twice, and then IAC was performed. The interval of each treatment of IVC was 3 weeks, and the regimen was the VEC regimen: Vincristine (< 3 years old: 0.05 mg/kg, ≥ 3 years old: 1.5 mg/m2, H44021772, Shenzhen Wanle Pharmaceutical, Shenzhen, China), Etoposide (< 3-years-old: 5 mg/kg, ≥ 3 years old: 150 mg/m2, H32025583; Jiangsu Hengrui Pharmaceutical, Jiangsu, China), and Carboplatin (< 3 years old: 18.6 mg/kg, ≥ 3-years-old: 560 mg/m2, H10920028; Qilu Pharmaceutical, Jinan, China). The IAC scheme was consistent with that in the IAC group. Before each operation, the eyes of both groups were photographed using the retcamii digital retinal camera (gsyjx [Jin] Zi 2006 No. 2220409, RetCam II; Massie, North Hollywood, CA, USA,) under general anesthesia. If the preoperative examination indicated that the treatment was not effective or was progressing, the patient was subjected to the enucleation of the eye. If the treatment was effective, the treatment was continued until the tumor achieved localized treatment conditions or was stable, with calcification and formation of scar tissue, and then into the follow-up period.
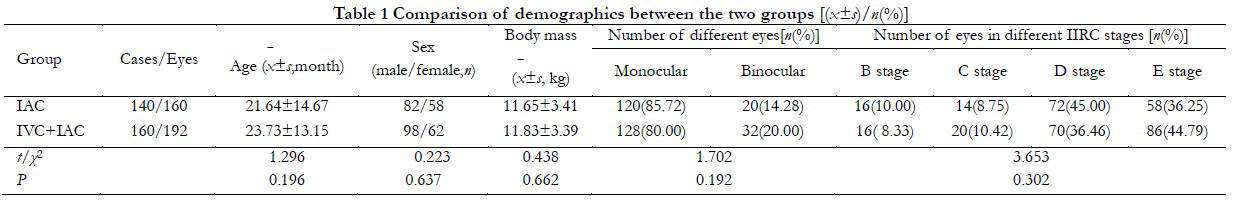
1.2.2 Therapeutic method (1) IAC method: the patient assumed the prone position, and anesthesia drug was given by intravenously injection. After routine disinfection, the femoral artery was punctured using Seldinger method, the blood vessel sheath of four F for Children was placed. Heparin (75 IU/kg) was then injected into the femoral artery for whole body heparinization. Using x-ray fluoroscopy, the affected internal carotid artery was selected using the 4FCobra super-slippery Catheter (Terumo, Shibuya City, Japan). The digital subtraction angiography of the internal carotid artery was performed with the manual push contrast medium (IOVEROL; Jiangsu Hengrui Pharmaceutical, Jiangsu, China; Figure 1A). After the ophthalmic artery was developed, the insertion line was marked, and 1.7f ev3.45°microcatheter was introduced using a cobra catheter. The super selective intubation of the ophthalmic artery was performed using a 45° microcatheter. The ophthalmic artery infusion chemotherapy was performed after angiography, confirmed, and locked into the position of the microcatheter (Fig. 1B). After dilution, the drug was perfused by microcatheter pulse perfusion at a rate of about 1 mL/min for 30 min. After perfusion, the artery sheath was removed, the puncture point was compressed for 5–10 min to stop the bleeding, and an elastic bandage was applied. After resuscitation, the lower limbs were immobilized and observed for 6 h. Fasting for 3 h and no water for 2 h after surgery was implemented. Low dose aspirin was given routinely for 2 weeks after surgery. In rare cases, when selective ophthalmic artery cannulation failed or was regurgitated due to a superior external carotid blood supply, downward angle of the ophthalmic artery opening, or extremely thin ophthalmic artery, the middle meningeal artery-ophthalmic artery approach or the vertebral artery-posterior cerebral artery-internal carotid artery-ophthalmic artery approach was used. If catheterization difficulties could not be resolved after the balloon occlusion of the distal external carotid artery at the ophthalmic artery ostium, perfusion of chemotherapeutic drugs through the ophthalmic artery was performed. (2) IVC method: blood routine examinations, blood biochemistry, hearing, and fundus oculi were examined before chemotherapy. The order of administration was the intravenous administration of Vincristine, hydration, and intravenous administration of Carboplatin and Etoposide. When creatinine clearance was less than 60 ml/min, the dose of Carboplatin was adjusted in accordance with glomerular filtration rate (GFR). (3) Local treatment: Laser photocoagulation/cryotherapy was used for local treatment. With the cooperation of the anesthesiology department, 810 nm or 532 nm semilaser conductors or condensers were used for the local irradiation of RB. Chemotherapy was applied within 48 hours following freezing to increase the concentration of chemotherapeutic drugs in vitreous, and the curative effect and the risk of metastasis was evaluated by ophthalmoscopy.
1.2.3 Follow-up and evaluation index After treatment, follow up was performed by telephone or clinic visit every 3 months during the first 2 years, every 6 months in the 3rd year after treatment, followed by every-year follow-up until June 30, 2020, and detailed general and ocular examinations [fundus photography or magnetic resonance imaging were performed. Telephone follow up was carried out by a same doctor and answered by the guardians of patients, and the questions included whether a patient had conjunctival congestion, eyelid swelling, forehead swelling, other physical symptom and recent vision, regular hospital visit, etc.
The primary outcomes for this study included technical success and eye-saving rate. The data regarding enucleation, recurrence, survival, and local or systemic complications were recorded and analysis. The success of operation (number of successful intubations/total number of intubations) and eye-saving rate (number of eyeballs retained/total number of eyes affected), recurrence (number of recurrent eyes/total number of eyes affected), survival (number of surviving children/total number of children affected), and complications were evaluated. Complications included conjunctival congestion, eyelid swelling, ptosis, forehead redness and swelling, eye bleeding, deep-set eyes, cataract, and bone marrow depression. The degree of myelosuppression was graded I–IV based on the criteria for WHO–toxicity scale A 5-grade system (0–4) for reporting of acute and subacute toxic effects of cancer treatment 10.
1.3 Statistical analysis
SPSS19.0 statistical software for Windows (SPSS, Chicago, IL, USA) was used for data analysis. The ages and weights of the sick children were verified to be normally distributed by the Shapiro-Wilk test. The differences in age and body weight between IAC and IVC + IAC groups were compared using independent-sample t-test. The difference in sex composition, eye difference, RB stage, eye-saving rate, RB recurrence, metastasis, mortality, and treatment-related complication between the two groups were compared by Chi-Square χ2 or Fisher’s exact test. The Kaplan-Meier survival analysis and log-rank test were used to evaluate the difference of survival rate between the two groups. A P<0.05 was considered statistically significance.
2 Results
2.1 Treatment-related general of the patients
Average 560 times of IAC was performed in 160 eyes of 140 patients with the 3.5 times for per eye in the IAC group. Four times of intubating were failure during IACs because of ophthalmic artery spasms with the success rate 99.28%. Average 384 times of IVC was carried out in 160 patients with the average 3.5 times for per person in the IVC + IAC group, and 540 times of ACV was performed in 192 eyes with 2.8 times for per eye. The intubating was failed in 3-time IACs with the success rate of 99.42%.
2.2 Comparison of eye preservation in different groups
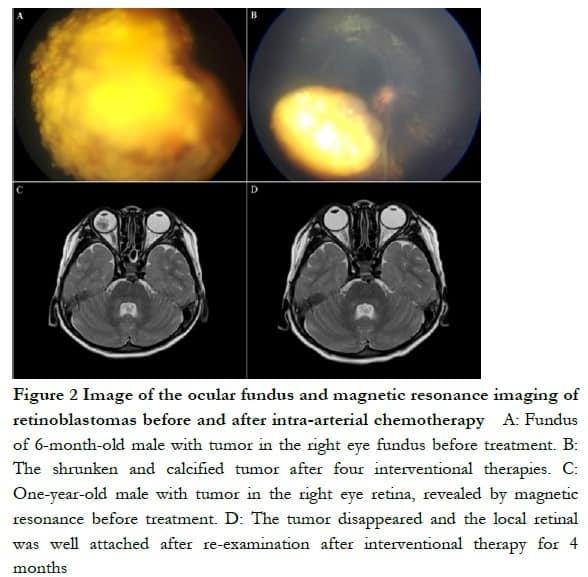
In the IAC group, eyeballs were successfully retained in 137 eyes with an eye-saving rate of 85.62%. A total of 23 eyes were enucleated, of which stage D RB was in 13 eyes and stage E in 10 eyes. A total of 38 eyes in the IVC + IAC group were enucleated, with an eye-saving rate of 81.25%. No significant difference was found in eye-saving rate between two groups (P > 0.05, Table 2). In patients with effective eyeball treatment, the tumor was significantly reduced or disappeared, and an MRI examination showed that the local retinal adhesion was good (Figure 2).
2.3 Comparison of the recurrence or metastasis rate of the two groups
All children were followed up for 2–60 months, with a median follow-up of 28.5 months. At the end of follow-up, 30 eyes, including 13 in stage D of RB and 17 in stage E of RB, recurred in the IAC group. The recurrence rate in the IAC group was 18.75% (30/160), which was significantly higher than that in the IVC + IAC group (10.93%, 21/192). The difference was statistically significant (χ2=4.299, P=0.038). Twelve cases of metastasis (including one case of distant organ multiple metastasis and six and five cases of stages E and D of RB central nervous system metastases, respectively) were observed in the IAC group, and five cases of central nervous system metastasis were observed in the IVC + IAC group. Metastasis rate of the IAC group was 8.57%, which was higher than that of the IVC + IAC group (3.75%). The difference was statistically significant (χ2=4.143, P=0.042).
2.4 Comparison of the survivals of the two groups
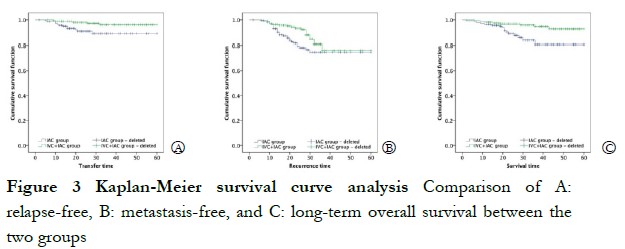
The 1-year survival rate was 96.43% and 98.75% in the IAC and IVC + IAC groups, respectively. No significant difference in the 1-year survival rate was observed between the two groups (χ2=1.766, P=0.184). At the end of follow up, the overall survival rate was 92.03%, and survival rate of the IAC group (88.57%, 124/140) was significantly lower than that of the IVC + IAC group (95.00%, 152/160; χ2=4.193, P=0.041). Sixteen children died in the IAC group during the follow up, including one case of intracranial hemorrhage, three cases of organ failure, one case of renal failure with distant metastasis, three cases of intracranial progression after refusing eyeball enucleation, three cases of intracranial progression after eyeball enucleation, and five cases of intracranial progression after chemotherapy. Eight children died in the IVC + IAC group, including three cases of organ failure after enucleation, one case of intracranial progression after eyeball enucleation, two cases of intracranial progression after refusing eyeball enucleation, and two cases of intracranial progression after stopping chemotherapy. The Kaplan-Meier univariate survival analysis showed significant differences in recurrence, metastasis, and long-term survival between the two groups (χ2 = 4.570, χ2 = 5.800, and χ2 = 7.771, respectively; P < 0.05; Figure 3).
2.5 Comparison of the complications between the two groups
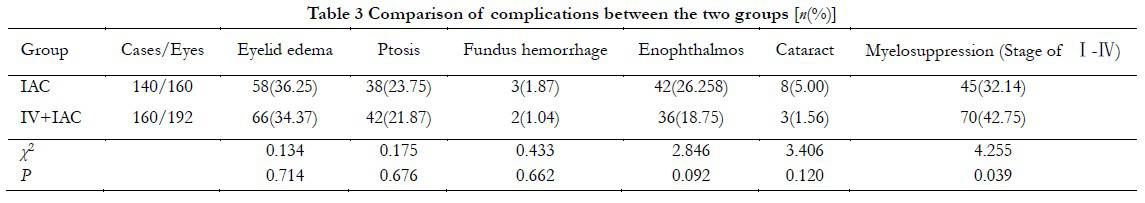
In the IAC group, 58, 38, 3, and 42 eyes had eyelid edema, ptosis, fundus hemorrhage, and enophthalmos, respectively. In the IVC + IAC group, 66, 42, 2, and 36 eyes had eyelid edema, ptosis, fundus hemorrhage, and enophthalmos, respectively. No significant difference was observed in the incidence of eyelid edema, ptosis, fundus hemorrhage, and enophthalmos between the two groups (P>0.05). Severe atrophy of enophthalmos occurred in one eye in the IAC group, and enucleation of the eyeball was performed. Short-term complications in most children returned to normal within 2 weeks, such as eyelid edema, ptosis, and fundus hemorrhage. Cataract occurred in eight eyes in the IAC group (5.00%), which was not significantly different from that in the IVC + IAC group (1.56%, 3/192; P<0.05). A total of 45 cases of total myelosuppression during chemotherapy in the IAC group was observed with an incidence of 32.14% (45/140), which was statistically significant in comparsin with in the IVC + IAC group (42.75%, 70/160; χ2=4.255, P=0.039). The myelosuppression of all children was improved after symptomatic treatment (Table 3).
3 Discussion
The comprehensive treatment based on IVC is a main method of eye protection therapy for children with RB. However, because patients with RB are often in stage D or E when diagnosed, the therapeutic effect of IVC on children with advanced intraocular RB is poor, and adverse reactions are serious 11. With the development of IAC technology, IAC has provided significantly higher eye preservation in the treatment of advanced RB, however, the recurrence rate still was higher within 1 year after treatment 12. The two treating methods have their own advantages and limitations. Shields et al 13 found that the eye preservation of stages D and E of RB were 67% and 50%, respectively, in the late stage (stage E) of RB treated with the combination of IVC and IAC. Ji et al 14 reported that IAC after whole body IVC had showed high preservation for late RB. Abramson et al 15 conducted a single center retrospective study of stage D RB treated with IAC and found that eye preservation using IAC as first-line treatment was higher than that of IAC as a second-line treatment. However, the subjects of these studies included children with failed IVC. Thus, this conclusion does not represent the real efficacy of IVC combined with IAC. Jiang et al 16 found that IVC combined with IAC and IAC alone for advanced RB did not reduce the overall survival, metastasis, and recurrence, but reduced the incidence of enophthalmos and cataract. The Kaplan-Meier analysis of Chen et al 17 also found that compared with IAC, IVC combined with IAC did not improve eye preservation and progression-free survival within 2 years in children with advanced RB. Xu et al 8 also reported that compared with IAC alone, IVC + IAC increased eye preservation and decreased recurrence, but no significant difference was found in metastasis and mortality. In the present study, by comparing the clinical efficacies of IVC combined with IAC and IAC alone for RB, the results showed no significant difference in eye preservation and 1-year survival between the IVC + IAC and IAC groups, which is consistent with the above results. However, the long-term follow up showed that the recurrence and metastasis for the IVC + IAC group were significantly lower than those in the IAC group and overall survival was significantly higher than that in the IAC group. The Kaplan-Meier survival analysis showed significant differences in recurrence, metastasis, and survival curves between the two groups. These results suggested that systemic IVC combined with IAC killed metastatic tumor cells, thus reducing the metastasis and recurrence of RB and improving the survival of children. However, the sample size was small, and the follow up time of some patients was short in this study. A multicenter, large-sample and extended follow up period study still is need to confirm the results of this study.
As an invasive treatment, the local infusion of melphalan and other drugs with high concentrations of IAC may cause the necrosis of arterial vascular epithelial cell or toxicity to intraocular nerves, resulting in local ocular complications, such as common fundus hemorrhage, and eyeball atrophy 19–20. The IAC also results in chronic injury of orbital vessels, leading to the atrophy of eyeball and retro-ocular fat because of the reduction of perieyeball and periorbital blood perfusion, and is prone to enophthalmos 21. At the same time, considering multiple drug resistance in IVC and IAC regimens 22, the melphalan combined with carboplatin or topotecan is used alternately in IAC. In this study, there is no significant difference in short-term complications, such as eyelid edema, ptosis, fundus hemorrhage, and enophthalmos between the IVC + IAC and IAC groups, and this finding is not completely consistent with the results of Jiang et al 16, which may be caused by the inclusion criteria used, and the sample size of the study. Carboplatin, a derivative of cisplatin, is a nonspecific drug affecting the cell cycle, which can cause ototoxicity, nephrotoxicity, and inhibition of the bone marrow hematopoietic system 23–24. In the present study, carboplatin was used both in IVC and IAC regimens, and the results showed that the incidence of myelosuppression in the IVC + IAC group was significantly higher than that in the IAC group. Therefore, the systemic chemotherapeutic drug concentration in children treated with IVC + IAC was speculated to be significantly higher than that treated with IAC.
In summary, compared with IAC alone, IVC combined with IAC for RB of children reduces the metastasis and recurrence and improved the survival of patients, but it also increases the incidence of systemic complications. Therefore, ophthalmologists and oncologists should adopt personalized treatment suitable for children based on different pathological characteristics of children to achieve the best clinical outcome and reduce the occurrence of adverse reactions.
Conflict of interest None declared.
Authors contribution Di Qi participated in the design of the research, drafting of the article, and final review and revision. Shen Gang and Zhang Jing participated in the design of the study, formulation of the treatment plan, and revision of the article. Shi Shengli and Lu Yuebing were responsible for the collection of research cases. Liu Jing and Hu Jing performed the statistical analysis and interpretation of research data. Chen Zhiping participated in the research topic selection and design, reviewed the accuracy, reliability, and completeness of the manuscript, and checked all aspects of the research.
References
[1]Rodriguez-Galindo C, Orbach DB, VanderVeen D. Retinoblastoma[J]. Pediatr Clin North Am, 2015, 62(1):201-223. DOI:10.1016/j.pcl.2014.09.014.
[2]Dimaras, H, Corson, TW. Retinoblastoma, the visible CNS tumor: a review[J]. J Neuro Res, 2019, 97:29-44. DOI:10.1002/jnr.24213.
[3]Shields CL, Manjandavida FP, Lally SE, et al. Intra-arterial chemotherapy for retinoblastoma in 70 eyes: outcomes based on the international classification of retinoblastoma[J]. Ophthalmology, 2014, 121(7):1453-1460. DOI:10.1016/j.ophtha.2014.01.026.
[4]Shields CL, Bas Z, Tadepalli S, et al. Long-term (20-year) real-world outcomes of intravenous chemotherapy (chemoreduction) for retinoblastoma in 964 eyes of 554 patients at a single centre[J]. Br J Ophthalmol, 2020, 104(11):1548-1555. DOI:10.1136/bjophthalmol-2019-315572.
[5]Munier FL, Mosimann P, Puccinelli F, et al. First-line intra-arterial versus intravenous chemotherapy in unilateral sporadic group D retinoblastoma: evidence of better visual outcomes, ocular survival and shorter time to success with intra-arterial delivery from retrospective review of 20 years of treatment[J]. Br J Ophthalmol, 2017, 101(8):1086-1093. DOI:10.1136/bjophthalmol-2016-309298.
[6]Chen Q, Zhang B, Dong Y, et al. Comparison between intravenous chemotherapy and intra-arterial chemotherapy for retinoblastoma: a meta-analysis[J/OL]. BMC Cancer, 2018, 18(1):486[2022-01-20]. http://pubmed.ncbi.nlm.nih.gov/ DOI: 10.1186/s12885-018-4406-6.
[7]Shields CL, Alset AE, Say EA, et al. Retinoblastoma Control With Primary Intra-arterial Chemotherapy: Outcomes Before and During the Intravitreal Chemotherapy Era[J]. J Pediatr Ophthalmol Strabismus, 2016, 53(5):275-284. DOI:10.3928/01913913-20160719-04.
[8]Fundus Disease Group of Chinese Ophthalmology Society, Ophthalmology Group of Chinese Ophthalmology Society, Ophthalmoplasty Orbital Group of Chinese Ophthalmology Society. Chinese guidelines for diagnosis and treatment of retinoblastoma (2019)[J] . Chinese Journal of Ophthalmology, 2019, 55(10):726-738. DOI:10.3760/cma.j.issn.0412-4081.2019.
[9]Liu Qinling. Diagnosis and treatment of retinoblastoma [M]. Beijing : People’s Medical Publishing House, 2015:1-183.
[10]Li Xingjun, Ma Xiaoli, Zhang Dawei, et.al. Side effects and prevention of high-dose cyclophosphamide-based chemotherapy in the treatment of high-risk neuroblastoma in children [J]. Chin J Appl Clin Pediatr, 2013, 28(2):141-145. DOI:10.3760/cma.j.issn.2095-428X. 02.019.
[11]Fabian ID, Onadim Z, Karaa E, et al. The management of retinoblastoma[J]. Oncogene, 2018, 37(12):1551-1560. DOI:10.1038/s41388-017-0050-x.
[12]Cui Xuehao, Ji Xunda, Zhao Peiquan. Research progress of transophthalmic artery interventional chemotherapy in the treatment of retinoblastoma [J]. Ophthalmology Times, 2018, 38(4):385-388. DOI:10.13389/j.cnki.rao.2018.0090.
[13]Shields CL, Kaliki S, Al-Dahmash S, et al. Management of advanced retinoblastoma with intravenous chemotherapy then intra-arterial chemotherapy as alternative to enucleation[J]. Retina, 2013, 33(10):2103-2109. DOI:10.1097/IAE.0b013e318295f783.
[14]Ji Xunda, Li Jiakai, Zhao Junyang, et.al. Observation on the efficacy of ophthalmic artery interventional chemotherapy after failure of intravenous chemotherapy for advanced intraocular retinoblastoma [J]. Chinese Journal of Ocular Fundus Diseases, 2015, 31(6):556-559. DOI:10.3760/cma.j.issn.1005-1015.2015.06.011
[15]Abramson DH, Daniels AB, Marr BP, et al. Intra-arterial chemotherapy (ophthalmic artery chemosurgery) for group D retinoblastoma[J/OL]. PLoS One, 2016, 11(1):e0146582[2022-02-02]. http://pubmed.ncbi.nlm.nih.gov/ DOI: 10.1371/journal.pone.0146582.
[16]Jiang Hua, Deng Hailang, Fang Qian, et al. Efficacy and safety of arterial chemotherapy and intravenous-arterial chemotherapy in the treatment of advanced intraocular retinoblastoma [J]. Chinese Journal of Interventional Radiology(Electronic Edition), 2018, 6(2):118-123. DOI:10.3877/cma.j.issn.2095-5782.2018.02.006.
[17]Chen Q , Zhang B , Dong Y , et al. Intravenous Chemotherapy Plus Intra-Arterial Chemotherapy Versus Intra-Arterial Chemotherapy in Patients with Advanced Retinoblastoma Cancer: A Retrospective, Multicentre-Based Study[J/OL]. Social Science Electronic Publishing. 2018-10-21. Available at SSRN: http://dx.doi.org/10.2139/ssrn.3271430
[18]Xu K, Liu J, Zhang C. Intra-arterial chemotherapy combined with VEC intravenous chemotherapy in the treatment of advanced retinoblastoma[J]. J BUON, 2020, 25(2):1199-1205.
[19]Gao Jingge. Systematic evaluation of the efficacy and safety of intra-ophthalmic artery chemotherapy for retinoblastoma [J]. Chinese Journal of Experimental Ophthalmology, 2016, 34(11):1031-1037. DOI:10.3760/cma.j.issn.2095-0160.2016.11.015.
[20]Jiang Hua, Fang Qian, Deng Hailang, et al. Clinical observation of ophthalmic artery infusion of chemical drugs in the treatment of retinoblastoma [J]. Chinese Journal of Ocular Fundus Diseases, 2017, 33(6):612-615. DOI:10.3760/cma.j.issn.1005-1015.2017.06.014.
[21]Tse BC, Steinle JJ, Johnson D, et al. Superselective intraophthalmic artery chemotherapy in a nonhuman primate model: histopathologic findings[J]. JAMA Ophthalmol. 2013;131(7):903-911. DOI:10.1001/jamaophthalmol.2013.2065.
[22]Chen M, Jiang H, Zhang J, et al. Outcome of intra-arterial chemotherapy for retinoblastoma and its influencing factors: a retrospective study[J]. Acta Ophthalmol, 2017,95(6):613-618. DOI:10.1111/aos.13333.
[23]Soliman SE, D’Silva CN, Dimaras H, et al. Clinical and genetic associations for carboplatin-related ototoxicity in children treated for retinoblastoma: a retrospective noncomparative single-institute experience[J/OL]. Pediatr Blood Cancer, 2018, 65(5):e26931[2022-02-16]. http://pubmed.ncbi.nlm.nih.gov/ DOI: 10.1002/pbc.26931.
[24]Oatess TL, Chen PH, Daniels AB, et al. Severe Periocular Edema after Intraarterial Carboplatin Chemotherapy for Retinoblastoma in a Rabbit (Oryctolagus cuniculus) Model[J]. Comp Med, 2020, 70(2):176-182. DOI:10.30802/AALAS-CM-18-000146.