Changes in conjunctival mucin expressions in patients with first diagnosis of dry eye and its clinical significance
Ouyang Weijie1, Liu Zuguo1, Sun Xuguang2, Deng Yingping3, Li Qingsong4, Huang Caihong1,
Lin Xiang1, Zhu Li1
1Xiamen University Affiliated Xiamen Eye Center, The Affiliated Xiang’an Hospital of Xiamen University and Eye Institute of Xiamen University, Fujian Provincial Key Laboratory of Ophthalmology and Visual Science, Xiamen 361102, China; 2Department of Ophthalmology, Beijing Tongren Hospital, Capital Medical University, Beijing 100069, China; 3Department of Ophthalmology, West China Hospital, Sichuan University, Chengdu 610044, China; 4Department of Ophthalmology, Putuo District Center Hospital, Shanghai 200062, China
Corresponding author: Liu Zuguo, Email: zuguoliu@xmu.edu.cn
[Abstract] [Download PDF in English] [Download PDF in Chinese] [Read Full Text]
Objective To investigate the expressions of MUC1, MUC4, MUC5AC and MUC16 in patients with first diagnosis of dry eye and their correlation with dry eye symptoms and signs.
Methods A cross-sectional study was conducted. Sixty-nine dry eye patients (69 eyes) as a dry eye group and 40 normal volunteers (40 eyes) as a normal volunteer group were recruited in Xiamen Eye Center of Xiamen University, Beijing Tongren Hospital, West China Hospital of Sichuan University and Shanghai Puotuo District Center Hospital from December 2016 to May 2018. Symptoms were evaluated by Chinese Dry Eye Questionnaire (CDEQ), Ocular Surface Disease Index (OSDI) and Dry Eye-Related Quality-of-Life Score Questionnaire (DEQS). Signs were assessed by tear film breakup time (TBUT), keratoconjunctival fluorescein sodium staining, and Schirmer I test. Conjunctival cells were collected by conjunctival impression cytology. The expression levels of MUC1, MUC4, MUC5AC and MUC16 mRNA in the two groups were determined by real-time fluorescence quantitative PCR. The correlation between the mRNA levels of conjunctival mucins and dry eye symptoms and signs were analyzed by Spearman correlation analysis. This study adhered to the Declaration of Helsinki. The study protocol was approved by the four Ethics Committees of Xiamen Eye Center of Xiamen University (No. 2017003), Beijing Tongren Hospital, Capital Medical University (No. TREC2016-29), West China Hospital of Sichuan University (No. 2016310) and Shanghai Puotuo District Center Hospital (No. PTEC-A-2016-18-1). Written informed consent was obtained from each subject before any medical examination.
Results The expression levels of MUC1 and MUC16 mRNA in dry eye patients were 3.277(0.568, 5.790) and 1.815(1.048, 3.694), which were higher than 1.055 (0.550, 2.010) and 1.024 (0.541, 1.965) in normal volunteer group (Z=819.00, P=0.008; Z=861.00, P=0.002). According to OSDI scores, MUC1 was mainly increased to 3.277(1.161, 6.226) in mild to moderate (12-32 points) dry eye patients (Z=9.04, P=0.029), and MUC16 was mainly increased to 1.968(1.074, 3.726) in severe (>32 points) dry eye patients (Z=12.24, P=0.007). MUC1 expression was positively correlated with TBUT, and was negatively correlated with corneal staining scoring and keratoconjunctival staining scoring (rs=0.270, P=0.025; rs=-0.331, P=0.006; rs=-0.325, P=0.007). MUC16 expression was positively correlated with TBUT, and was negatively correlated with blurred vision scoring, quality of life scoring (reading, driving at night, computer and TV use, etc.) (rs=0.249, P=0.039; rs=-0.359, P=0.047; rs=-0.370, P=0.034; rs=-0.558, P=0.016; rs=-0.498, P=0.006; rs=-0.515, P=0.002).
Conclusions The gene expressions of MUC1 and MUC16 are higher in dry eye patients. MUC1 expression is related to patients’ signs. MUC16 expression is related to the quality of life of patients.
[Key words] Dry eye; Mucin; MUC1; MUC16
Fund program: National Key R&D Program of China (2018YFA0107304); National Natural Science Foundation of China (81870627, 81900825)
DOI: 10.3760/cma.j.cn115989-20211128-00652
Dry eye is a chronic ocular surface disease caused by multiple factors. Patients may experience a series of uncomfortable symptoms such as eye dryness and foreign body sensation, which significantly affect their quality of life 1. The core mechanism of dry eye is tear film instability 2-3. Mucin, an essential component of the tear film, plays an important role in maintaining the stability of the tear film by lubricating tissues, clearing foreign substances, and fighting against bacteria 4. Mucin in ocular tissues can be divided into transmembrane and secreted types, mainly secreted by the conjunctiva, cornea, and lacrimal gland 5. Abnormalities in their quality or quantity can lead to decreased tear film stability, causing tears to be unable to stay on the ocular surface and triggering dry eye 6, which can further cause damage to the ocular surface epithelial cells, worsening the abnormality of mucin. Therefore, monitoring changes in the ocular surface mucin is an important area of dry eye research. Several studies have examined the changes in mucin content in dry eye patients, but the reported results vary. Some studies have found that dry eye patients have a reduced number of goblet cells in the ocular surface, decreased levels of MUC5AC protein 7-9, and reduced expression of MUC1, MUC4, and MUC16 in the conjunctival epithelium 10-12. However, other studies have found that the expression levels of MUC1 and MUC16 in the ocular surface and tears of patients with both dry and non-dry syndromes increased 13-15. Although the latest version of the international dry eye disease workshop of the International Tear Film and Ocular Surface Society summarized all the research results comprehensively, it still cannot accurately conclude the changes in mucin in the ocular surface tissue of dry eye patients 16. This study aims to explore the changes in mucin expression in the conjunctival tissue of dry eye patients and their association with dry eye symptoms and signs, providing a reference for the development of dry eye prevention and treatment measures.
1 Information and methods
1.1 Baseline data
A cross-sectional, multicenter study was conducted from December 2016 to May 2018 at Eye Institute and Affiliated Xiamen Eye Center of Xiamen University, Beijing Tongren Hospital, Sichuan Huaxi Hospital and Shanghai Puotuo District Center Hospital. A total of 69 patients (69 eyes) who were diagnosed with dry eye for the first time according to Chinese expert consensus on dry eye (2013) 6 were enrolled in DECS-C. These patients had not received any dry eye-related medication intervention before their initial visit. A total of 40 normal volunteers (40 eyes) were included as the normal volunteer group. The average age of the dry eye group was (39.7±13.3) years, while the average age of the subjects in the normal volunteer group was (22.2±2.8) years. There was no significant correlation between the expression levels of MUC1, MUC4, MUC5AC, MUC16 and age in the dry eye group (MUC1: rs=-0.065, P=0.598; MUC4: rs=-0.014, P=0.911; MUC5AC: rs=-0.247, P=0.081; MUC16: rs=-0.029, P=0.815). The male-to-female ratio in the dry eye group was 1:2, while it was 7:3 in the normal volunteer group. The relative expression levels of MUC1 in males and females of the dry eye group were 2.96 (0.80, 4.05) and 2.39 (0.01, 5.67), respectively. The relative expression levels of MUC4 in males and females were 2.02 (0.91, 3.96) and 2.50 (1.09, 4.90), respectively. The relative expression levels of MUC5AC in males and females were 0.21 (0.02, 1.13) and 0.06 (0.02, 3.41), respectively. The relative expression levels of MUC16 in males and females were 2.20 (1.20, 3.80) and 1.67 (0.99, 3.62), respectively. There were no statistically significant differences in the expression levels of various mucins between different genders (Z=522.0, P=0.94; Z=486.5, P=0.69; Z=184.0, P=0.84; Z=457.5, P=0.37). The enrolled dry eye patients were from Xiamen Eye Center of Xiamen University (27 cases), Beijing Tongren Hospital of Capital Medical University (20 cases), Zhongshan Hospital of Putuo District, Shanghai (19 cases), and West China Hospital of Sichuan University (3 cases). Among them, patients from Beijing had a relatively lower relative expression level of MUC1 compared to other regions (Z=35.78, P=0.0001), while patients from Xiamen had lower tear secretion and tear breakup time (TBUT) compared to other regions (Z=20.68, P=0.0001; Z=19.91, P=0.0001). There were no significant regional differences in other indicators. Due to the small number of patients (only 3 cases) from Sichuan, potential bias may exist, therefore, a comparative analysis between these 3 patients from Sichuan and patients from other regions was not conducted at this stage.
Inclusion criteria for dry eye patients were as follows: (1) aged 18 years or older, (2) first-time outpatient visitors, and (3) patients with symptoms and signs consistent with the Chinese diagnostic criteria for dry eye 6. Exclusion criteria included: (1) previously diagnosed and treated dry eye patients, (2) individuals with a history of hypersensitivity to fluorescein sodium solution, (3) patients with other ocular diseases aside from dry eye or those who had undergone any eye surgeries, (4) individuals with a history of eye medication use, (5) patients with a history of systemic diseases or systemic medication use, and (6) patients deemed unsuitable for participation in this study by the principal investigator and/or assisting researchers. Both eyes of eligible patients were examined, with the eye exhibiting higher dry eye symptom scores selected as the study eye. If both eyes had equal dry eye scores, the eye with more severe corneal fluorescein staining was chosen. In cases where both eyes had equal levels of fluorescein staining, the right eye was selected as the study eye.
Inclusion criteria for normal volunteers were as follows: (1) aged 18 years or older, (2) no ophthalmic diseases requiring treatment, and (3) at least one eye meeting the following criteria: (a) absence of dry eye symptoms, (b) tear film breakup time (TBUT) exceeding 5 seconds, (c) unanesthetized Schirmer test result > 5 mm/5 min, and (d) absence of corneal fluorescein staining. Exclusion criteria included: (1) diagnosed with dry eye or presence of suspected dry eye symptoms or signs, (2) history of ophthalmic diseases, (3) individuals with a history of hypersensitivity to fluorescein sodium, and (4) individuals deemed unsuitable for participation in this study by the principal investigator and/or assisting researchers.
Both eyes were examined by ophthalmology, and the symptoms were scored using the Chinese Dry Eye Questionnaire (CDEQ), and the one eye with a high symptom score was used as the research eye. If the scores of both eyes were equal, the heavier eye with corneal fluorescein sodium staining was included; If the degree of sodium fluorescein staining of the cornea of both eyes was comparable, the eye with a shorter TBUT was included; If the TBUT was equal, the right eye was taken as the study eye. The whole research procedure was performed following the Declaration of Helsinki and was approved by the Medical Ethics Committee of Xiamen University Affiliated Xiamen Eye Center, Beijing Tongren Hospital, West China Hospital and Putuo District Center Hospital (2017003, TREC2016-29, 2016(310), PTEC-A-2016-18-1). Written informed consent was obtained from all participants.
1.2 Methods
1.2.1 Quality Control The four eye centers standardized and unified calibration of examination equipment before the project started, and the inspectors had GCP certificates and unified training, including examination items, examination methods, diagnostic criteria, ethics, etc. A Clinical Research Coordinator (CRC) was provided by a third-party company to monitor the quality of the study, track the progress of the study, and coordinate the work of the trial.
1.2.2 Eye Examination Clinical examinations were conducted on all participants, including recording patient medical history (duration of VDT use), dry eye symptom scoring, TBUT measurement, corneal and conjunctival fluorescein staining scoring, and Schirmer test I. The examinations were performed on the participants in the following sequence as part of this study.
1.2.2.1 Dry Eye Questionnaires The symptoms of dry eye were evaluated by the Chinese Dry Eye Questionnaire (CDEQ), Ocular Surface Disease Index (OSDI) and Dry Eye-Related Quality-of-Life Score Questionnaire (DEQS). All patients completed the questionnaires independently under the supervision and guidance of the investigator. According to the symptom scores obtained from the OSDI questionnaire, the dry eye group was categorized into different symptom groups based on the following criteria: a total score ≤ 12 indicated the asymptomatic group, consisting of 9 eyes; a total score > 12 and ≤ 32 indicated the mild to moderate symptom group, consisting of 40 eyes; a total score > 32 indicated the severe symptom group, consisting of 20 eyes 19.
1.2.2.2 TBUT Measurement A fluorescein sodium test strip moistened with one drop of saline solution was placed in contact with the lower conjunctival sac of the participant. The participant was instructed to blink several times to ensure a thorough mixing of the strip with tear fluid. The participant was then instructed to look straight ahead, and the time from their last blink to the appearance of the first tear film breakup point was observed using a 16x slit lamp microscope (SL220, Carl Zeiss, Germany) with cobalt blue light. A TBUT value of less than 5 seconds was considered abnormal. The test was performed three times, and the average value was recorded.
1.2.2.3 Determination of Corneal Staining Scores After completing the TBUT measurement, corneal fluorescein staining was observed under a 16x slit lamp microscope with cobalt blue light. The integrity of the corneal epithelium was evaluated according to the grading standards of the International Ophthalmology Institute 17. The cornea was divided into five regions: superior, nasal, central, inferior, and temporal. Each region was scored from 0 to 3, and the total score was the sum of scores for all regions, with a maximum score of 15. A total score of ≥1 indicated an abnormality. The Van Bijsterveld grading system 18 was used for keratoconjunctival staining (KC) to evaluate the integrity of the corneal epithelium. The cornea was divided into temporal, central, and nasal regions. Each region was scored from 0 to 3, and the total score was the sum of scores for all regions, with a maximum score of 9. A total score of ≥1 indicated an abnormality.
1.2.2.4 Schirmer I Test Without local anesthesia, the folded portion of the Schirmer test strip was placed in the outer one-third of the patient’s lower conjunctival sac. The patient was instructed to look straight ahead and then close their eyes. After 5 minutes, the test strip was removed, and the length of tear fluid infiltration was measured. A tear fluid infiltration length of ≤5 mm was considered abnormal.
1.2.2.5 Evaluation of the severity of dry eye according to dry eye symptoms and signs In terms of symptoms, an OSDI score of ≤32 is classified as mild to moderate dry eye, while a score >32 indicates severe dry eye. In terms of signs, dry eye is classified as mild or severe based on the presence or absence of corneal staining.
1.2.3 Conjunctival impression cytology specimen collection Eye drops containing proparacaine hydrochloride were instilled to provide surface anesthesia. The patient was instructed to look downwards. With one hand, the examiner gently lifted the upper conjunctiva of the eye being examined, while using tweezers without teeth in the other hand to grasp a cellulose acetate membrane. The cellulose acetate membrane was lightly applied to the upper conjunctiva of the patient and pressed gently several times in a uniform manner. After 3-5 seconds, the cellulose acetate membrane was removed using tweezers without teeth, and the membrane containing conjunctival cells was placed into 350 μl preservation solution. The preservation solution was composed of a lysis solution in a volume ratio of 100:1 with β-mercaptoethanol. The specimen was vortexed for 1 minute to fully lyse the cells and then stored at -80°C. After the procedure, the eye being examined was instilled with ofloxacin antibiotic eye drops.
1.2.4 Real-time fluorescence quantitative PCR method was used to determine the relative expression levels of MUC1, MUC4, MUC5AC and MUC16 mRNA in the specimens Following the operating procedures of RNA extraction kit (DP420, Tiangen Biotech (Beijing) Co., Ltd.), the lysate containing imprint cells stored at -80℃ was dissolved at room temperature, vortexed and mixed uniformly, transferred onto CS filtration column, centrifuged at 12,000 rpm for 2 minutes with a radius of 10 cm, and the filtrate was collected. Then, 70% ethanol was added at a volume of 1:1 (v/v), mixed with a pipette, and all solutions and precipitates were transferred to CR1 adsorption column. The tube cover was gently placed and centrifuged at 12,000 rpm for 30-60 seconds, and the waste liquid was discarded. After adding 350 μl of protein removal solution RW1 to the CR1 adsorption column, it was centrifuged at 12,000 rpm for 30-60 seconds, and the waste liquid in the collection tube was discarded. Then, 80 μl of DNase I working solution was added to the CR1 adsorption column, and kept at room temperature for 15 minutes, followed by the addition of 350 μl of protein removal solution RW1. After centrifugation at 12,000 rpm for 30-60 seconds, the waste liquid in the collection tube was discarded. After adding 500 μl wash solution RW to the CR1 adsorption column, it was left at room temperature for 2 minutes and then centrifuged at 12,000 rpm for 30-60 seconds. The waste liquid in the collection tube was discarded, and the washing process was repeated twice. After centrifugation at 12,000 rpm for 2 minutes, the waste liquid was discarded. The CR1 adsorption column was left at room temperature for several minutes to thoroughly dry the residual wash solution in the adsorbent material. Then, the CR1 adsorption column was placed into a new RNase-free centrifuge tube, 14 μl of RNase-free ddH2O was added to the center of the membrane, covered with the tube cover gently, left at room temperature for 2 minutes, and then centrifuged at 12,000 rpm for 1 minute to obtain RNA solution. The experiment was conducted using an RNA reverse transcription kit (KIT0204, Thermo Fisher Scientific Corporation, USA). The total system volume was 20 μl, including 4 μl of 5-fold reaction buffer, 2 μl of dNTP, 1 μl of random primer, 1 μl of RevertAid H Minus reverse transcriptase and 12 μl of RNA solution. The reaction was carried out at 25℃ for 10 minutes, 42℃ for 60 minutes, and 70℃ for 10 minutes to obtain cDNA. PCR was performed using a SYBR pre-mix kit (RR420A, Takara Corporation, Japan), with a reaction volume of 10.0 μl, including 1.0 µl of cDNA, 0.2 µl of forward and reverse primers, 5.0 µl of mixed solution and 3.6 µl of DEPC water. PCR reaction conditions: denaturation at 95℃ for 10 seconds, annealing at 60℃ for 5 seconds, extension at 72℃ for 20 seconds, for a total of 45 cycles. The primer sequences (5′-3′) were as follows: MUC1 forward sequence was ACAATTGACTCTGGCCTTCC, the reverse sequence was CAGACTGGGCAGAGAAAGGA; MUC4 forward sequence was CTTACTCTGGCCAACTCTGTAGTG, the reverse sequence was GAGAAGTTGGGCTTGACTGTC; MUC5AC forward sequence was TCCACCATATACCGCCACAGA, reverse sequence was TGGACCGACAGTCACTGTCAAC; MUC16 forward sequence was GCCTCTACCTTAACGGTTACAATGAA, reverse sequence was GGTACCCCATGGCTGTTGTG; β-actin forward sequence was CATGTACGTTGCTATCCAGGC, reverse sequence was CTCCTTAATGTCACGCACGAT. The relative expression levels of various mucin genes were calculated using the 2-ΔΔCt method with β-actin as the internal reference.
1.3 Statistical analysis
Statistical analysis was performed using SPSS 17.0 software. The normality of the continuous data was assessed using the Shapiro-Wilk test, and as they did not follow a normal distribution, they were expressed as M(Q1, Q3). The Wilcoxon rank-sum test was used to compare the differences in assessment indicators between the two groups, while the Kruskal-Wallis H test was used for multiple-group comparisons. Multiple comparisons were conducted using the Nemenyi test. The Spearman rank correlation analysis was used to evaluate the association between the expression level of conjunctival mucins in dry eye patients and the scores of ocular symptoms and signs. A p-value <0.05 was considered statistically significant.
2 Results
2.1 Comparison of relative expression levels of MUC1, MUC4, MUC5AC, and MUC16 mRNA in the conjunctiva of dry eye group and control group subjects
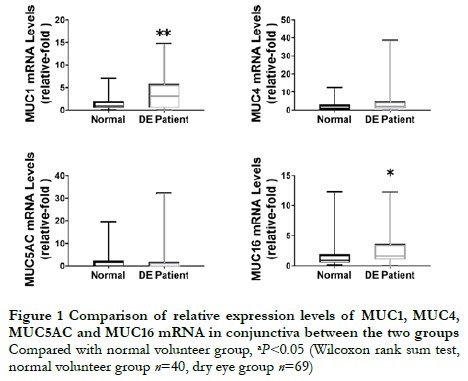
The relative expression levels of transmembrane mucins MUC1 and MUC16 mRNA in the conjunctiva of dry eye patients were 3.277 (0.568, 5.790) and 1.815 (1.048, 3.694), respectively, which were higher than those in the normal volunteers, which were 1.055 (0.550, 2.010) and 1.024 (0.541, 1.965), respectively. The differences were statistically significant (Z=819.00, P=0.008; Z=861.00, P=0.002). The relative expression level of MUC4 mRNA in the conjunctiva of dry eye patients was 2.465 (1.016, 4.881), which was higher than that in the normal volunteers, which was 1.356 (0.371, 3.082), but the difference was not statistically significant (Z=1,029.00, P=0.055). The relative expression level of MUC5AC mRNA in the conjunctiva of dry eye patients was 0.075 (0.022, 1.725), which was not statistically significantly different from that in the normal volunteers, which was 0.288 (0.075, 2.325) (Z=438.00, P=0.226) (Figure 1).
2.2 Comparison of clinical symptom scores between the dry eye patients and the normal volunteers
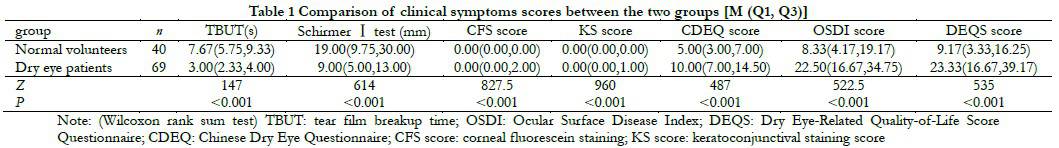
The dry eye group had significantly lower tear break-up time (TBUT) and average tear secretion volume compared to the normal volunteer group. The corneal staining score, conjunctival staining score, Chinese Dry Eye Questionnaire (CDEQ) score, Ocular Surface Disease Index (OSDI) score, and Dry Eye Questionnaire Score (DEQS) were all higher in the dry eye group compared to the normal volunteer group. The differences were statistically significant (all P<0.05) (Table 1). Among dry eye patients, there were 28 cases with mild symptoms and signs, 10 cases with severe symptoms but mild signs, 21 cases with mild symptoms but severe signs, and 10 cases with both severe symptoms and signs.
2.3 Association between the expression levels of MUC1, MUC4, MUC5AC, and MUC16 mRNA in the conjunctiva and dry eye signs in dry eye patients
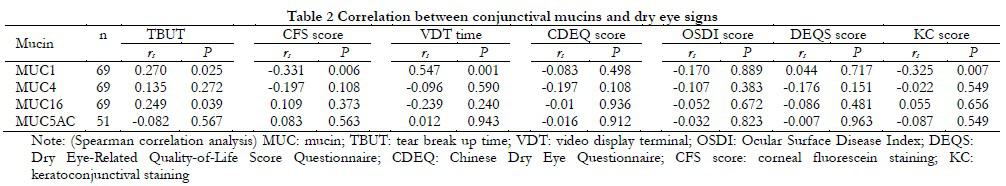
The relative expression level of MUC1 mRNA in the conjunctiva was positively correlated with tear breakup time (TBUT) (rs=0.270, P=0.025), and negatively correlated with corneal staining score (rs=-0.331, P=0.006) and conjunctival staining score (rs=-0.325, P=0.007), and positively correlated with visual display terminal (VDT) use time (rs=0.0547, P=0.001). The relative expression levels of MUC1, MUC4, MUC5AC, and MUC16 mRNA were not correlated with tear secretion (rs=-0.184, P=0.134, rs=0.066, P=0.596, rs=-0.230, P=0.108, rs=-0.117, P=0.343) (Table 2).
2.4 Association between the expression levels of MUC1, MUC4, MUC5AC, and MUC16 mRNA in the conjunctiva and the severity of dry eye symptoms in dry eye patients
The relative expression level of MUC1 mRNA in the conjunctiva was positively correlated with tear breakup time (TBUT) (rs=0.270, P=0.025), and negatively correlated with corneal staining score (rs=-0.331, P=0.006) and conjunctival staining score (rs=-0.325, P=0.007), and positively correlated with the duration of visual display terminal (VDT) use (rs=0.0547, P=0.001). However, the relative expression levels of MUC1, MUC4, MUC5AC, and MUC16 mRNA were not correlated with tear secretion (rs=-0.184, P=0.134, rs=0.066, P=0.596, rs=-0.230, P=0.108, rs=-0.117, P=0.343) (Table 2).
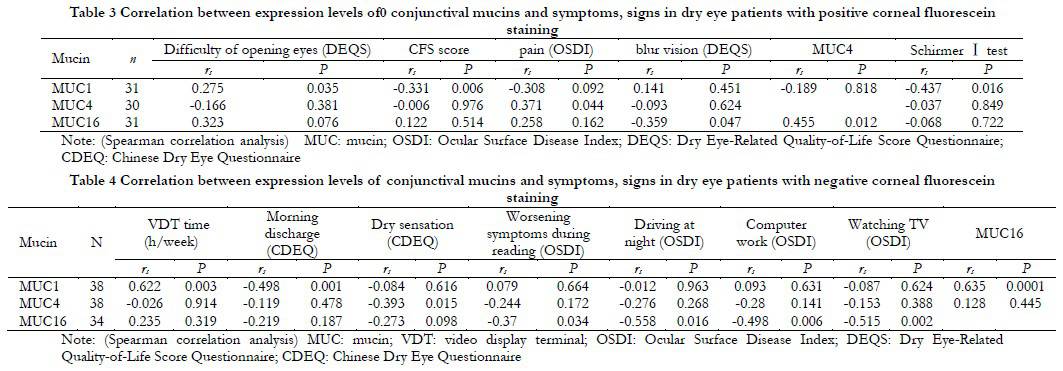
Among patients with positive corneal staining, the relative expression level of MUC1 mRNA was positively correlated with the difficulty of opening eyes symptom score in DEQS (rs=0.275, P=0.035), and negatively correlated with corneal staining score and tear secretion (rs=-0.331, P=0.006; rs=-0.437, P=0.016). The expression of MUC4 was positively correlated with eye pain score (rs=0.371, P=0.044). The relative expression level of MUC16 mRNA was negatively correlated with blurred vision symptom score in DEQS (rs=-0.359, P=0.047) (Table 3). The expression of MUC4 was positively correlated with the expression of MUC16 (rs=0.455, P=0.012). Among patients with negative corneal staining, the expression of MUC1 was positively correlated with VDT time (rs=0.622, P=0.003), and negatively correlated with frequency of morning discharge score (rs=-0.498, P=0.001), and positively correlated with MUC16 (rs=0.635, P<0.001). The expression of MUC4 was negatively correlated with dryness symptom score (rs=-0.393, P=0.015); MUC16 was negatively correlated with quality of life scores in dry eye patients, such as worsening symptoms during reading, impact on driving at night, impact on computer work, and impact on watching TV (rs=-0.370, P=0.034; rs=-0.558, P=0.016; rs=-0.498, P=0.006; rs=-0.515, P=0.002) (Table 4).
2.5 Comparison of relative expression levels of conjunctival MUC1, MUC4, MUC5AC, and MUC16 mRNA in dry eye patients with different degrees of symptoms and signs
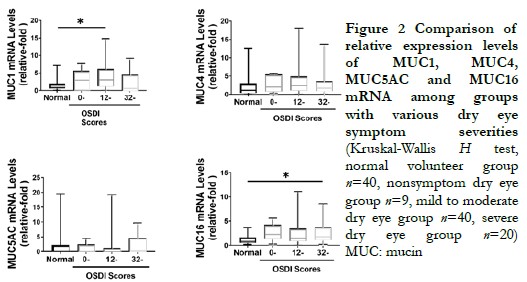
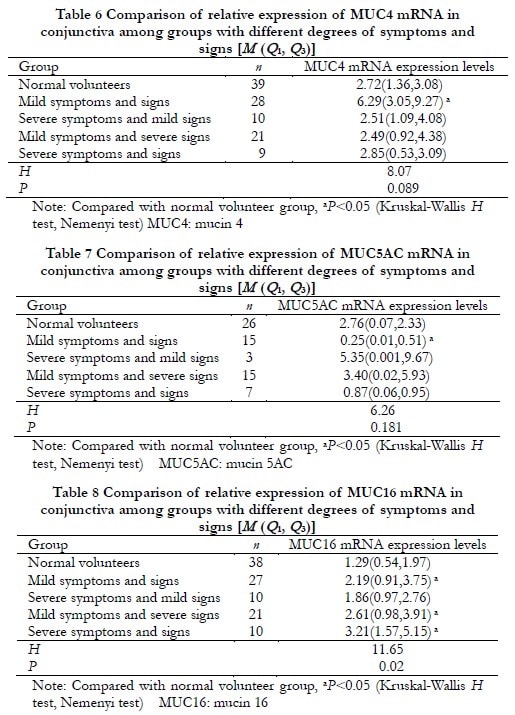
In patients with mild symptoms and signs, the relative expression levels of MUC1, MUC4, and MUC16 mRNA were significantly increased compared to the normal volunteer group (Z=245, P<0.001; Z=342, P=0.009; Z=366, P=0.022), while the relative expression level of MUC5AC mRNA was significantly decreased compared to the normal volunteer group (Z=106, P=0.015). The differences were statistically significant. In patients with severe symptoms but mild signs, the relative expression level of MUC1 mRNA was significantly increased compared to the normal volunteer group (Z=112, P=0.039), and the difference was statistically significant. In patients with mild symptoms but severe signs, as well as in patients with severe symptoms and signs, the relative expression levels of MUC16 mRNA were significantly increased compared to the normal volunteer group (Z=236, P=0.010; Z=80, P=0.004). The differences were statistically significant. However, there were no statistically significant differences in the expression levels of MUC1, MUC4, and MUC5AC genes compared to the normal volunteer group (all P>0.05) (Table 5-8).
3 Discussion
Studies have shown that pathological changes in dry eyes are accompanied by alterations in ocular surface mucins 16. The results of this study revealed that dry eye patients exhibited increased expression levels of transmembrane mucins, MUC1 and MUC16 mRNA, compared to normal individuals. Additionally, the expression level of MUC1 mRNA was significantly correlated with tear break-up time (TBUT) and corneal staining scores. Research suggests that transmembrane mucins play roles in lubrication, anti-inflammation, antimicrobial activity, and hydrophilic properties 20. It has been reported that the release of inflammatory mediators can promote elevated expression of MUC1. Upregulated MUC1 expression can inhibit inflammation by suppressing the Toll-like receptor pathway, and MUC1 and MUC16 regulate ocular surface inflammation by inhibiting interleukin 6, interleukin 8, and tumor necrosis factor-alpha 21-23. The enrolled patients in this study primarily consisted of individuals with mild to moderate dry eye. We speculate that in the early stages of dry eye, the function of conjunctival epithelial cells remains intact, and the microenvironment of the ocular surface is not completely imbalanced. The body is under stress due to dry eye stimulation and mild inflammation, and conjunctival epithelial cells may undergo stress-induced overexpression of mucins to resist ocular surface irritation and early inflammation 13,20.
Changes in transmembrane mucins in dry eye have been unclear, and the results vary greatly. One of the main reasons is that the inclusion criteria for patients in these studies are not completely consistent. Some studies included patients with systemic factors such as dry syndrome 14, while others included both initial and follow-up dry eye patients 13. Due to inconsistent inclusion criteria, the comparability is poor. The expression of mucins in patients who have already received dry eye treatment has been affected, such as by preservatives in eye drops 24. Therefore, only patients without systemic diseases affecting the ocular surface and without previous ocular surface medication can reflect the true expression of mucins in patients. This study is the first to investigate the expression of conjunctival mucins in initial visit dry eye patients without systemic diseases, which better reflects the original pathological changes in dry eye.
MUC1 is the smallest mucin but has many functions. MUC1 facilitates the distribution of mucin layers and is believed to be a key mucin in the formation of a mucosal barrier on the surface of mucosal cells. Loss of MUC1 makes the mucosal epithelium susceptible to microbial infection 25 and increases dye permeability. It has also been reported that mice lacking MUC1 have a higher incidence of conjunctivitis and blepharitis 26. Therefore, MUC1 plays an important role in maintaining the normal microenvironment of the ocular surface. In this study, Spearman rank correlation analysis revealed that the expression of MUC1 was positively correlated with TBUT and negatively correlated with corneal and conjunctival staining scores, indicating that the expression of MUC1 affects the severity of dry eye signs.
MUC16 is a transmembrane mucin located on the apical membrane of corneal and conjunctival epithelial cells and plays an important role in antimicrobial defense, maintaining corneal barrier function, and signal transduction 27. Some studies have found increased expression of MUC16 in dry eye patients 13,15. Upregulation of MUC16 is considered a protective signal released by ocular surface epithelial cells under stress 28. This study found increased expression of MUC16 in the conjunctival tissue of dry eye patients. Spearman rank correlation analysis revealed a negative correlation between MUC16 expression and the quality of life in dry eye patients, reflecting the protective role of MUC16.
There are also some limitations in this study. The relative expression level of MUC5AC detected by the assay was low, and in some samples, the MUC5AC sequence could not be detected, which may be related to partial RNA degradation caused by delayed freezing after sample collection. After sample collection, the samples were temporarily placed in ice bags and then taken back to the laboratory for freezing preservation after the clinic ended. Such storage conditions may affect the experimental results. Secondly, most of the initial visit dry eye patients had mild to moderate dry eye, with very few severe dry eye patients, so the number of severe dry eye patients was small, which may introduce bias, and further studies with larger sample sizes are needed. In addition, due to limited conditions, impression cell collection could not meet the sample concentration requirements for laboratory protein detection. Therefore, the protein levels of mucins in dry eye patients were not measured, and only the four most commonly studied mucins were selected as research targets at the gene level. With the continuous advancement of tear protein detection methods, tear proteomics will provide us with a more comprehensive analysis at the protein level in the future 29, which will be the focus of further research.
In conclusion, this study collected data from initial visit dry eye patients, showing increased expression of mucins MUC1 and MUC16 in dry eye. The expression of MUC1 is associated with dry eye signs, and the expression of MUC16 is associated with patient quality of life.
Conflict of Interest The relationship between the funder and the ADES/principal investigator/assisting investigator in planning, conducting and reporting the research does not affect the results of the research and its interpretation. The rights and interests of participants can be guaranteed.
Author Contribution Liu Zuguo, Deng Yingping, Sun Xuguang: topic selection, research design and standardization, review of data analysis results, revision and finalization of the intellectual content of the paper; Ouyang Weijie: implementation of the research, collection and analysis of experimental data, writing and modification of the paper; Li Qingsong, Huang Caihong, Lin Xiang, Zhu Li: implementation of the research, parameter measurement, experimental data collection and analysis, and paper modification
Acknowledgements Thanks to Santen Corporation of Japan for providing financial support for this study
References
[1] Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report[J]. Ocul Surf, 2017, 15(3):276-283. DOI: 10.1016/j.jtos.2017.05.008.
[2] Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report[J]. Ocul Surf, 2017, 15(3):438-510. DOI: 10.1016/j.jtos.2017.05.011.
[3] Zhang X, M VJ, Qu Y, et al. Dry eye management: targeting the ocular surface microenvironment[J/OL]. Int J Mol Sci, 2017, 18(7):1398[2022-05-16]. http://www.ncbi.nlm.nih.gov/ pubmed/28661456. DOI: 10.3390/ijms18071398.
[4] Hong J. Paying close attention to advanced researches of ocular surface mucin and significance in the diagnosis and management of dry eye[J]. Chin J Exp Ophthalmol, 2020, 38(11):910-915. DOI: 10.3760/cma.j.cn115989-20200715- 00499.
[5] Zhang C, Fang ZJ, Zhao SZ. Mucins and ocular surface disease[J]. Int Rev Ophthalmol, 2021, 45(6): 540-545. DOI: 10.3760/cma.j.issn.1673-5803.2021.06.014.
[6] 中华医学会眼科学分会角膜病学组. 干眼临床诊疗专家共识(2013年)[J]. 中华眼科杂志, 2013, 49(1):73-75. DOI: 10.3760/cma.j.issn.0412-4081.2013.020.
[7] Zhang J, Yan X, Li H. Analysis of the correlations of mucins, inflammatory markers, and clinical tests in dry eye[J]. Cornea, 2013, 32(7):928-932. DOI: 10.1097/ICO.0b013e3182801622.
[8] Moore JE, Vasey GT, Dartt DA, et al. Effect of tear hyperosmolarity and signs of clinical ocular surface pathology upon conjunctival goblet cell function in the human ocular surface[J]. Invest Ophthalmol Vis Sci, 2011, 52(9):6174-6180. DOI: 10.1167/iovs.10-7022.
[9] Zou XR, Lu LN, Xu Y, et al. Comparison of tear index and tear film function between type 2 diabetic patients and normal subjects[J]. Chin J Exp Ophthalmol, 2019, 37(10):814-819. DOI: 10.3760/cma.j.issn.2095-0160.2019.10.009.
[10] Argüeso P, Balaram M, Spurr-Michaud S, et al. Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjögren syndrome[J]. Invest Ophthalmol Vis Sci, 2002, 43(4):1004-1011.
[11] Shimazaki-Den S, Dogru M, Higa K, et al. Symptoms, visual function, and mucin expression of eyes with tear film instability[J]. Cornea, 2013, 32(9):1211-1218. DOI: 10.1097/ICO.0b013e318295a2a5.
[12] Corrales RM, Narayanan S, Fernández I, et al. Ocular mucin gene expression levels as biomarkers for the diagnosis of dry eye syndrome[J]. Invest Ophthalmol Vis Sci, 2011, 52(11):8363-8369. DOI: 10.1167/iovs.11-7655.
[13] Gipson IK, Spurr-Michaud SJ, Senchyna M, et al. Comparison of mucin levels at the ocular surface of postmenopausal women with and without a history of dry eye[J]. Cornea, 2011, 30(12):1346-1352. DOI: 10.1097/ICO.0b013e31820d852a.
[14] Caffery B, Heynen ML, Joyce E, et al. MUC1 expression in Sjogren’s syndrome, KCS, and control subjects[J]. Mol Vis, 2010, 16:1720-1727.
[15] Caffery B, Joyce E, Heynen ML, et al. MUC16 expression in Sjogren’s syndrome, KCS, and control subjects[J]. Mol Vis, 2008, 14:2547-2555.
[16] Willcox M, Argüeso P, Georgiev GA, et al. TFOS DEWS II tear film report[J]. Ocul Surf, 2017, 15(3):366-403. DOI: 10.1016/j.jtos.2017.03.006.
[17] Lemp MA. Report of the National Eye Institute/Industry workshop on clinical trials in dry eyes[J]. CLAO J, 1995, 21(4):221-232.
[18] van Bijsterveld OP. Diagnostic tests in the Sicca syndrome[J]. Arch Ophthalmol, 1969, 82(1):10-14. DOI: 10.1001/archopht.1969.00990020012003.
[19] The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007)[J]. Ocul Surf, 2007, 5(2):93-107. DOI: 10.1016/s1542-0124(12)70082-4.
[20] Uchino Y. The ocular surface glycocalyx and its alteration in dry eye disease: a review[J]. Invest Ophthalmol Vis Sci, 2018, 59(14):DES157-DES162. DOI: 10.1167/iovs.17-23756.
[21] Kato K, Lillehoj EP, Lu W, et al. MUC1: the first respiratory mucin with an anti-inflammatory function[J/OL]. J Clin Med, 2017, 6(12):110[2022-06-06]. http://www.ncbi.nlm.nih.gov/ pubmed/29186029. DOI: 10.3390/jcm6120110.
[22] Albertsmeyer AC, Kakkassery V, Spurr-Michaud S, et al. Effect of pro-inflammatory mediators on membrane-associated mucins expressed by human ocular surface epithelial cells[J]. Exp Eye Res, 2010, 90(3):444-451. DOI: 10.1016/j.exer.2009.12.009.
[23] Menon BB, Kaiser-Marko C, Spurr-Michaud S, et al. Suppression of Toll-like receptor-mediated innate immune responses at the ocular surface by the membrane-associated mucins MUC1 and MUC16[J]. Mucosal Immunol, 2015, 8(5):1000-1008. DOI: 10.1038/mi.2014.127.
[24] Kim JR, Oh TH, Kim HS. Effects of benzalkonium chloride on the ocular surface of the rabbit[J]. Jpn J Ophthalmol, 2011, 55(3):283-293. DOI: 10.1007/s10384-011-0008-4.
[25] McAuley JL, Linden SK, Png CW, et al. MUC1 cell surface mucin is a critical element of the mucosal barrier to infection[J]. J Clin Invest, 2007, 117(8):2313-2324. DOI: 10.1172/JCI26705.
[26] Kardon R, Price RE, Julian J, et al. Bacterial conjunctivitis in Muc1 null mice[J]. Invest Ophthalmol Vis Sci, 1999, 40(7):1328-1335.
[27] Perez BH, Gipson IK. Focus on molecules: human mucin MUC16[J]. Exp Eye Res, 2008, 87(5):400-401. DOI: 10.1016/j.exer.2007.12.008.
[28] Yazu H, Kozuki N, Dogru M, et al. The effect of long-term use of an eyewash solution on the ocular surface mucin layer[J/OL]. Int J Mol Sci, 2019, 20(20):5078[2022-06-12]. http://www.ncbi.nlm.nih.gov/pubmed/31614909. DOI: 10.3390/ijms20205078.
[29] Zhou L, Liu DN. Paying attention to proteomics of human tear: clinical significance and application in ocular surface diseases[J]. Chin J Exp Ophthalmol, 2016, 34(2):97-102. DOI: 10.3760/cma.j.issn.2095-0160.2016.02.001.