·Clinical Science·
Effects of short-term use of atropine with different concentrations and frequencies on eye safety in children
He Meinan, Mi Baoyue, Zhu Ying, Liu Lin, Zhang Ziyu, Du Bei, Wei Ruihua
Tianjin Key Laboratory of Retinal Functions and Diseases, Tianjin Branch of National Clinical Research Center for Ocular Disease, Eye Institute and School of Optometry, Tianjin Medical University Eye Hospital, Tianjin 300384, China
Corresponding author: Wei Ruihua, Email: rwei@tmu.edu.cn
[Abstract] [Download PDF in English] [Download PDF in Chinese] [Read Full Text]
Objective To evaluate the effect of short-term topical administration of atropine eye drops with various concentrations and frequencies on eye safety in children.
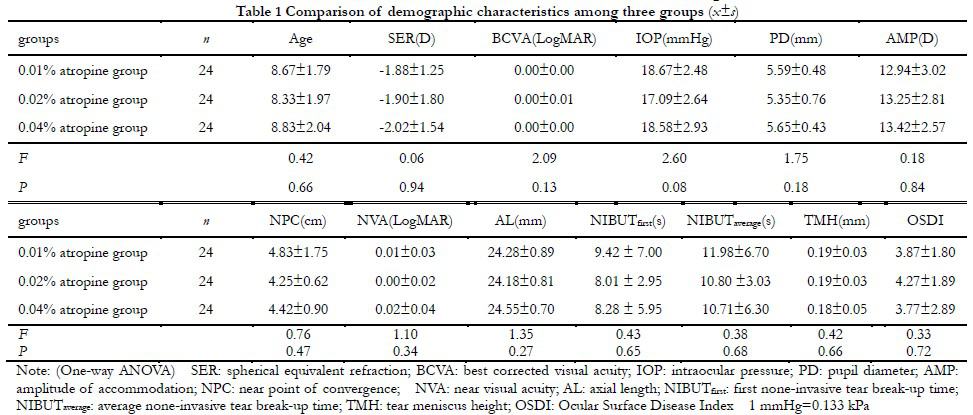
Methods A double-blind randomized controlled trial was conducted. Seventy-two children with ametropia or pre-myopia (72 eyes) were enrolled in Tianjin Medical University Eye Hospital from December 2020 to January 2022. The subjects were randomly divided into 0.01% atropine group,0.02% atropine group and 0.04% atropine group according to a random number table, 0.02% Atropine Group and 0.04% Atropine Group according to a random number table ,with 24 cases (24 eyes) in each group. Automatic refraction with an automatic computer optometry device, subjective refraction with a phoropter, intraocular pressure with a non-contact tonometer, axial length by optical biometrics, the amplitude of accommodation (AMP) with the push-up method, pupil diameter with pupilometer, near visual acuity at 33 cm with a standard logarithmic visual acuity chart, tear evaluation with Keratograph 5M and Ocular Surface Disease Index (OSDI) questionnaire survey were performed among all subjects. One drop of 0.01%, 0.02%, and 0.04% atropine was administrated to the subjects according to grouping. One drop of 0.01%, 0.02%, and 0.04% Atropine was administrated to the subjects according to grouping, and the pupil diameter was measured every 10 minutes until the pupil did not enlarge three times, then the data after a single treatment of the three groups were recorded. After one-week application of the corresponding concentration of atropine eye drops once at night, the data after one-week treatment were recorded. For the next week, the application frequency of 0.01% and 0.02% atropine groups changed to once daily in the morning and evening, and 0.04% atropine group maintained once at night, then the data after two-week treatment were recorded. Data of the right eyes were analyzed. Changes in pupil diameter, AMP and other parameters before and after treatment of the three groups were compared.
Results Pupil diameters of 0.01%, 0.02% and 0.04% atropine groups were (5.59±0.48), (5.35±0.76), (5.65±0.43)mm before treatment respectively, (7.00±0.68), (7.17±0.58) and (8.40±1.71)mm after a single treatment, (6.67±0.62), (6.56±0.65) and (7.60±0.69)mm after one-week treatment, (6.96±0.49), (7.04±0.53) and (7.60±0.36)mm after two-week treatment. There were significant differences in pupil diameter at different time points after treatment among the three groups (Fgroup=9.430, P<0.001; Ftime=156.620, P<0.001). The AMP of 0.01%, 0.02% and 0.04% atropine groups were (12.94±3.02), (13.25±2.81) and (13.42±2.60)D before treatment respectively, (11.62±2.61), (11.53±2.06) and (9.64±1.93)D after a single treatment, (11.14±2.61), (11.33±2.33) and (8.30±1.18)D after one-week treatment, (9.99±1.81), (8.72±1.25) and (8.76±2.12)D after two-week treatment. There was no significant difference in the AMP among the three groups (Fgroup=2.800, P=0.063). In the three groups, the AMP at different time points after treatment were significantly lower than that before treatment (Ftime=61.400, P<0.001). There was no difference in spherical equivalent refraction, intraocular pressure, near visual acuity, axial length, first none-invasive tear break-up time, average none-invasive tear break-up time, tear meniscus height and OSDI score among the three groups (Fgroup=0.030, 0.630, 1.420, 0.580, 0.140, 0.120, 0.340, 0.142; all P>0.05). There were significant differences in spherical equivalent refraction, intraocular pressure, first none-invasive tear break-up time, average none-invasive tear break-up time, tear meniscus height and OSDI score at different time points before and after medication among the three groups (Ftime=12.560, 4.730, 4.720, 5.220, 3.720; all P<0.05).
Conclusions Varying pupil dilation and AMP reduction occur after the use of different concentrations of atropine and are more severe at higher concentrations. Increased administration frequency of atropine is associated with more pupil dilation and AMP reduction, but there is no intolerable adverse effect.
[Key words] Atropine; Administration and dosage; Pupil diameter; Amplitude of accommodation
Fund program: Social Science Major Project of Tianjin Education Commission (2020JWZD20)
DOI: 10.3760/cma.j.cn115989-20220519-00230
Myopia has become a global public health problem, according to the epidemiological analysis of myopia, it is expected that by 2050, the global overall myopia prevalence will reach 49.8%, of which high myopia will reach 9.8% 1-2. According to the reports, in 2020, the overall myopia rate of Chinese children and adolescents is 52.7%, and the myopia rate of primary, middle and high schools is 35.6%, 71.1% and 80.5%, respectively, and the incidence of highly myopic-related eye diseases is also gradually increasing 3-4. The prevention and control of myopia has attracted great attention from visual health experts around the world 5-6. At present, the prevention and control methods of myopia are varied, including orthokeratology, defocus frame glasses, soft contact lens with peripheral defocus design and drugs. A number of clinical studies and Meta-analyses have shown that low concentration atropine eye drops are one of the effective ways to prevent and control myopia 7-8. Studies have shown that the effect of atropine eye drops on myopia control is concentration-dependent, but the higher the mass concentration of atropine eye drops after withdrawal, the more serious the myopia rebound, and it is believed that 0.01% atropine eye drops can maintain the myopia control effect while the incidence of adverse reactions is lower 9-10. A randomized controlled study on Chinese children also proved that 0.01% atropine eye drops have good effectiveness and safety in the prevention and control of myopia 11. A study on myopia control in children in Hong Kong, China, showed that 0.05% atropine eye drops were more effective in myopia control than 0.01% atropine, but their effect on pupils and regulation was slightly greater than 0.01% atropine 12. Some studies have also shown that reducing 0.02% atropine eye drops frequency is equivalent to increasing 0.01% atropine frequency in the prevention and control of myopia in children, and the degree and incidence of adverse reactions have no significant change 13. However, the conclusions of current studies on the methods and effects of atropine eye drops dosage and administration frequency are still not completely clear. The purpose of this study was to investigate the effects of different concentration and administration frequency of atropine on children’s eye parameters and safety, and to provide reference for the formulation of myopia prevention and control program of atropine eye drops.
1 Data and methods
1.1 General Information
A randomized controlled double-blind clinical trial was conducted to continuously include 72 children with myopia or pre-myopia who were treated in the Tianjin Medical University Eye Hospital from December 2020 to January 2022. Inclusion criteria: (1) Age 6~12 years old, spherical equivalent refraction (SER) was among 0.00~ -6.00D; (2) Normal astigmatism ≤1.50D, inverse astigmatism ≤0.75D; (3) Best corrected visual acuity (BCVA) not less than 0.1 (LogMAR); (4) In the past, only single-focus frame glasses were used to correct refractive errors; (5) There were no other organic eye lesions except refractive errors; (6) No muscarinic receptor antagonists were used in the past 90 days. Exclusion criteria: (1) Patients who used low-concentration atropine eye drops or orthokeratology and other myopia prevention and control methods; (2) Patients who cannot complete all the follow-up. Both eyes were required to meet the inclusion criteria, and the right eye was selected as the study eye. Subjects were randomly divided into 0.01% atropine group, 0.02% atropine group and 0.04% atropine group by random number table, with 24 eyes in each group. There was no significant difference in baseline data among three groups (all P>0.05) (Table 1). This study followed the Declaration of Helsinki, and the study protocol was reviewed and approved by the Ethics Committee of Tianjin Medical University Eye Hospital [Approval number: 2020KY(L)-51]. All subjects and their guardians were fully aware of the implementation method and purpose of this study before entering the study cohort, and voluntarily signed informed consent.
1.2 Methods
1.2.1 Preparation and distribution of atropine eye drops
0.01%, 0.02% and 0.04% of atropine eye drops are provided by Shenyang Xingqi Eye Medicine Co., LTD. The package of the drops is identical and the drops are put into three black sealed bags respectively with the drug concentration marked on the outside of the bags, and the drugs are distributed according to the results of the group. The eye drops are kept and distributed by the same doctor. Only the physician who dispenses the drug knows the concentration.
1.2.2 Methods of Medication
According to the group, the pupil diameter of each group was measured once every 10 minutes after one drop of atropine with the corresponding concentration was applied to both eyes respectively. When the pupil diameter did not change for three times, the eye parameters of each group were re-measured and recorded as the data after a single administration. At the first week, each group was given atropine eye drops with corresponding concentration once a night in both eyes. At the second week, 0.01% atropine and 0.02% atropine groups changed the frequency of administration to once a day in the morning and once a day in the night, and 0.04% atropine group was maintained once a night.
1.2.3 Methods of ophthalmic examination and evaluation indexes
Subjects in each group were examined before medication, after a single dose, 1 week after medication and 2 weeks after medication, respectively. All examinations are completed between 8:00 am and 11:00 am. The procedure for each examination method is as follows: (1) The diopter was measured under the normal pupil. Firstly, the Automatic computer optometer (KR-800, Japan TOPCON Company) was used to measure both eyes respectively. Each eye was measured 3 times, and the average value was taken, with the error of each measurement not exceeding -0.50D. After that, the same experienced optometrist used a comprehensive optometrist to perform subjective optometry according to the standard optometry procedure, and recorded spherical equivalent refraction (SER) and the best corrected visual acuity (BCVA); (2) Pupil diameter (PD) was measured with an objective pupil measuring instrument (SN-M000716, OASIS Company, USA) under fixed illumination, the subject was instructed to look directly at the visual mark in the measuring instrument, then, doctors could read the pupil diameter according to the scale in the measuring instrument, and measured for three times to obtain the average value; (3) Amplitude of accommodation (AMP) was measured by propulsive method in the state of full correction. Subjects were asked to focus on a single line of visual markers above the best vision on the near vision card, gradually move the near vision card, immediately inform the tester when persistent blurring occurred, and record the location of the vision card. The reciprocal of the distance from this position to the inspected eye was AMP, and the measurement was repeated for 3 times to obtain the average value; (4) Near visual acuity (NVA) was measured in the distance of 33 cm, with full correction, the standard logarithmic near visual acuity table was used to measure the opposite eye by blocking the eye plate, and the subject read the opening direction of the visual label successively until two visual labels were misread in the same line. The visual acuity value corresponding to the preceding line is the NVA of the eye; (5) For optical biometrics, the Lenstar-900 (Heck-Streit AG, Switzerland) was used. During the measurement, subjects were asked to look at the small red dot in the front. The axial length (AL) was measured three times per eye, and the measurement error was no more than 0.05mm. (6) The tear surface was evaluated with a non-invasive ocular surface integrated analyzer (Keratograph 5M, OCULUS, Germany), which included the first invasive tear break up time (NIBUTfisrt), average noninvasive tear break up time (NIBUTaverage) and tear meniscus height (TMH); (7) The Standard Ocular Surface Disease Index (OSDI) questionnaire was improved on the basis of the OSDI questionnaire developed by the International Dry Eye Group. It includes 21 questions related to ocular fatigue, ocular discomfort, photophobia, ocular dryness, physical discomfort, etc. Each question has 4 options, never, occasionally, often, and frequently were recorded as 0, 1, 2, and 3 points respectively, and the total score was calculated according to the number of questions answered by the subject and the score for each question: Total OSDI score = the sum of all scores×25/ the number of questions answered, the higher the OSDI value, the more severe the symptoms. All subjects and guardians filled in the questionnaire under the detailed explanation of the doctor, and designated the past week as the recall period. Before the experiment, 10 children aged 6-12 were selected to fill in the questionnaire with the help of doctors and guardians, and the questionnaire was repeated twice. The total difference between the two scores was not more than 2 points, and the questionnaire results were highly reliable.
1.3 Statistical Methods
SPSS 24.0 (IBM) was used for statistical analysis. The measurement data were confirmed by Shapiro-Wilk test to be consistent with normal distribution, expressed as x(_)±s. ANOVA was used to compare the baseline data, the time required for mean pupil dilation to maximum and the difference of maximum pupil diameter among all groups. The overall differences of SER, IOP, AMP, NVA and PD among all groups before medication, after a single dose, 1 week after medication and 2 weeks after medication were compared by Two-way repeated measures ANOVA, and multiple comparisons were performed by Tukey-Kramer test. Two-tail test was used, and P<0.05 was considered statistically significant.
2 Results
2.1 PD comparison of subjects in each group after single administration
After a single dose, the maximum PD of were (7.00±0.68), (7.17±0.58) and (8.40±1.71) mm in 0.01% atropine group, 0.02% atropine group and 0.04% atropine group, respectively, and the overall difference was statistically significant (F=11.280, P < 0.001). PD of all subjects decreased varying degrees after 10 min, and pupils of subjects gradually dilated from 20 min (Table 2). The average pupil dilation time of 0.01% atropine group, 0.02% atropine group and 0.04% atropine group were (48.75±12.27), (50.83±7.76) and (52.92±8.59) min, respectively, and there was no statistical significance in overall comparison (F=1.099, P=0.339). The maximum PD expansion from baseline were (1.41±0.59), (1.81±0.55) and (2.75±1.64) mm in 0.01% atropine group, 0.02% atropine group and 0.04% atropine group, respectively, and the overall difference was statistically significant (F=11.620, P<0.001).
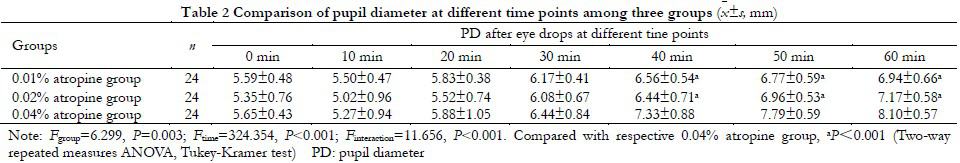
2.2 Comparison of PD after medication in each group
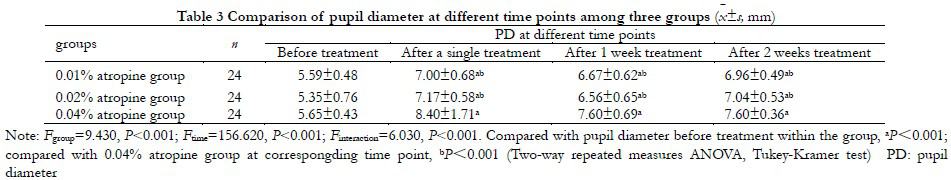
Overall comparison of PD among all groups showed statistically significant differences (Fgroup=9.430, P<0.001). PD in 0.04% atropine group was significantly greater than that in 0.01% atropine group and 0.02% atropine group after single administration, 1 week with administration and 2 weeks with administration, with statistically significant differences (all P<0.001) (Table 3). The
difference of PD at different time points before and after medication was statistically significant (Ftime=156.620, P<0.001). PD after single drop, 1 week of treatment and 2 weeks of treatment was significantly increased in all groups compared with that before treatment, with statistical significance (all P<0.001) (Table 3).
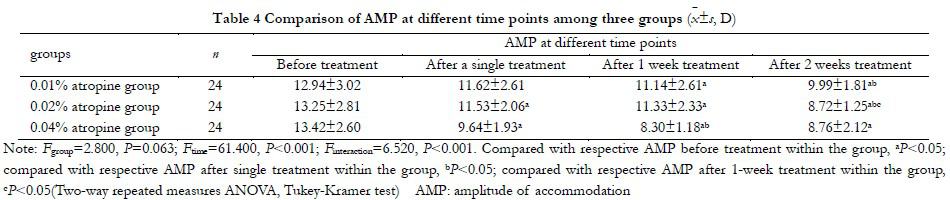
2.3 Comparison of AMP before and after medication in each group
There was no significant difference in the overall comparison of AMP among all groups (Fgroup=2.800, P=0.063), and there was significant difference in the overall comparison of AMP at
different time points before and after medication (Ftime=61.400, P<0.001). AMP in 0.02% atropine group after 2 weeks of treatment was significantly lower than that after 1 week of treatment, with statistical significance (P<0.05) (Table 4).
2.4 Comparison of SER, IOP, NVA and AL before and after treatment in each group
There was no statistically significant difference in the overall SER comparison among all groups (Fgroup=0.030, P=0.967), but there was statistically significant difference in the overall SER comparison among different time points (Ftime=12.560, P<0.001). Farsighted shift was observed in all groups as the treatment time increased. The difference was statistically significant (all P<0.01) in 0.02% atropine group at 1 week and 2 weeks compared with before treatment; the difference was statistically significant in 0.04% atropine group at 2 weeks compared with before treatment, The difference was statistically significant (P=0.016) (Table 5).
There was no significant difference in the overall comparison of IOP among the three groups (Fgroup=0.630, P=0.533), but there was significant difference in the overall comparison of IOP between the three groups at different time points before and after medication (Ftime=4.730, P=0.003). IOP in 0.02% atropine group after 1 and 2 weeks of treatment was significantly lower than that after single treatment, with statistical significance (all P<0.05) (Table 6).
The overall comparison of NVA between the three groups showed no statistical significance (Fgroup=1.420, P=0.245), and the overall comparison between different time points before and after medication showed no statistical significance (Ftime=1.190, P=0.313) (Table 7).
There was no significant difference in AL between the three groups at different time points before and after treatment (Fgroup=0.580, P=0.561; Ftime=0.590, P=0.623) (Table 8).
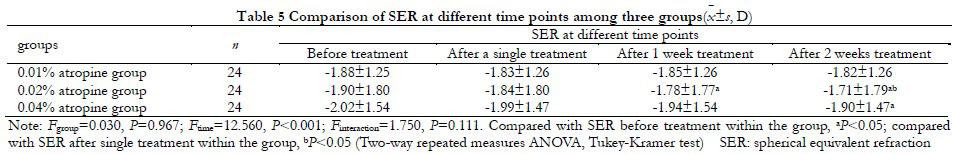
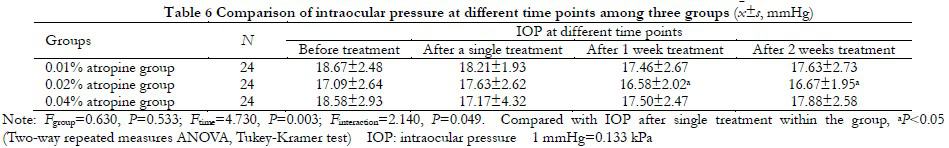

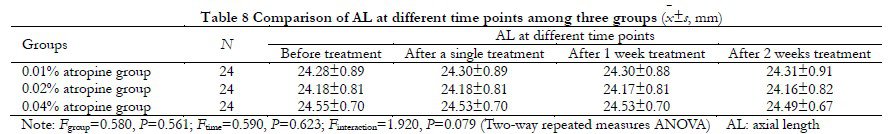
2.5 Comparison of tear function and OSDI scores in each group
There was no significant difference in NIBUTfirst among the three groups (Fgroup=0.140, P=0.871). There was significant difference in NIBUTfisrt at different time points before and after treatment (Ftime=4.720, P=0.003). NIBUTfirst in 0.04% atropine group at 2 weeks of treatment was significantly longer than that before treatment and after single treatment, with statistical significance (all P<0.05) (Table 9).
There was no significant difference in NIBUTaverage among the three groups (Fgroup=0.120, P=0.890). The overall difference of NIBUTaverage at different time points before and after medication was statistically significant (Ftime=5.220, P=0.002). NIBUTfirst in 0.04% atropine group at 2 weeks of treatment was significantly longer than that after single treatment and after 1 week of treatment, with statistical significance (all P < 0.01) (Table 10).
There was no significant difference in TMH between the three groups (Fgroup=0.340, P=0.716). There were statistically significant differences in overall TMH comparison at different time points after medication (Ftime=3.720, P=0.012) (Table 11).
There was no significant difference in overall OSDI score among the three groups (Fgroup=0.142, P=0.868). The overall OSDI score was significantly different at different time points before and after treatment (Ftime=6.882, P=0.002). OSDI score of all groups after 1 week of treatment was increased to different degrees compared with before treatment, and OSDI score of 0.04% atropine group after 1 week of treatment was significantly higher than before treatment, the difference was statistically significant (P=0.012). After 2 weeks of treatment, OSDI scores in all groups recovered to the level before treatment (Table 12).
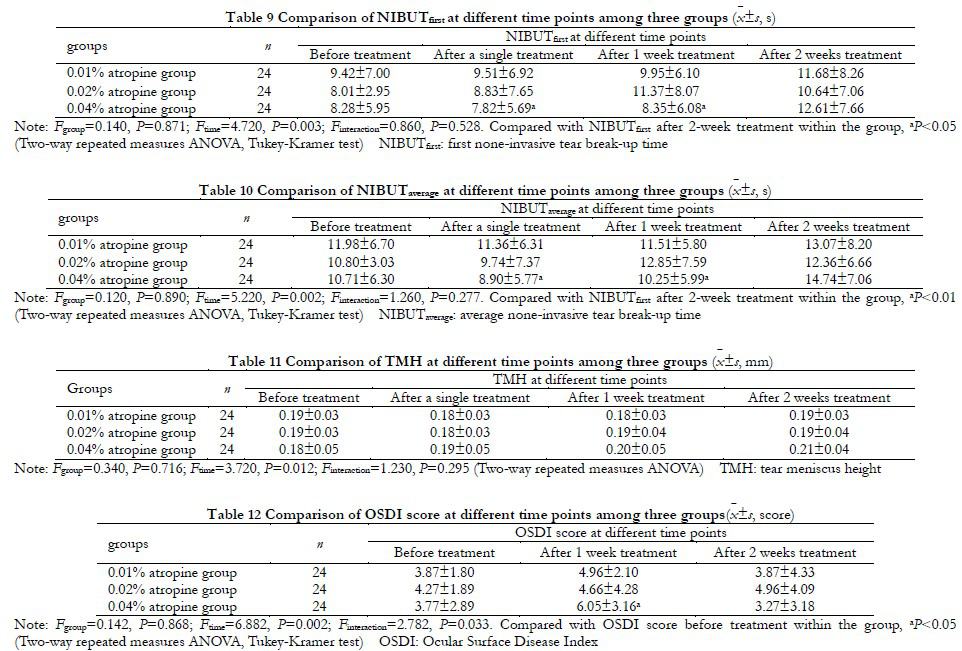
3 Discussion
The ATOM studies showed that 0.01% atropine eye drops reduced the adverse effects of high concentration atropine eye drops, while maintaining myopia control 9-10. The expert guide also clarified 0.01% atropine as a common drug for the prevention and control of myopia 14. However, recent studies have found that 0.01% atropine is not effective in controlling axial growth in some myopic children 15-16. So how to balance the control effect and adverse reactions of atropine drops on myopia has become a hot topic discussed by clinical experts. Studies have shown that 0.05% atropine is twice as effective as 0.01% atropine in controlling myopia in children, and its effect on pupils and regulation is slightly higher than 0.01% atropine eye drops 12,17.
Studies on effects of different concentrations of atropine eye drops on children’s eye parameters can provide evidence support for future clinical studies of atropine eye drops of different concentrations. In this study, 0.01%, 0.02%, 0.04% atropine eye drops were used to evaluate the changes of children’s eye parameters after single administration, continuous administration and increasing the frequency of administration. In previous studies, optical biometrics were used to evaluate pupil diameter, and the repeatability of measurement results was often poor. In this study, SN-M000716 OASIS, an objective pupillometer, was used in a fixed lighting environment (indoor 300~500 lx), and the observation window was equipped with a ruler. The results obtained were objective and systematic errors were reduced.
The results of this study showed that the higher the concentration of atropine, the longer the time required for pupil dilation after a single dose; At the same time, the pupil was briefly narrowed after a single dose, which was consistent with the results of our previous study on the effect of 0.01% atropine on adult PD 18. Chen et al. 19 found that 0.05% anisodamine also had the same effect on the pupil, which may be related to the pharmacological mechanism of M receptor antagonist. This study showed that the myosis of 0.02% atropine group and 0.04% atropine group was larger than that of 0.01% atropine group 10 minutes after a single administration, suggesting that it is possible to estimate the children’s response to atropine by observing the reduction of PD after a single administration, so as to predict the effectiveness of atropine in myopia control.
The results of this study showed that after a single dose of 0.01% atropine group, the average PD dilation was about 1.41 mm, and the AMP reduction was about 1.32 D. Previous studies in our group have shown that after 0.01% atropine was used in adults at a single point, the dilation of PD was about 1.5 mm, and the decrease of AMP was 1.2 D 18. Mydriasis was more pronounced in adults than in children after a single dose of 0.01% atropine eye drops, but the reduction of AMP was less. In the 0.01% atropine group, after continuous administration for 1 week, the dilation of PD was 1.08mm, which was less than the value after a single administration, possibly related to the gradual adaptation of the drug, and the reduction of AMP was 1.8D, which was greater than the value after a single drop, indicating the accumulation effect of continuous administration, which is consistent with the results of Fu et al. 20. After the frequency of 0.01% atropine was changed to 2 times a day for 1 consecutive week, the dilation of PD was 1.37mm, which was not significantly different from the PD after a single dose, but larger than the value after 1 week of medication; while the reduction of AMP was 2.95 D, which was significantly larger than the value after a single dose and 1 consecutive week of medication, indicating the accumulation effect of drugs. The dilation of PD in 0.02% atropine group was about 1.82 mm after a single administration, which was greater than 1.21 mm after 1 week of continuous administration, and the reduction of AMP was about 1.72 D, which was lower than 1.93 D after 1 week of continuous administration, indicating the accumulation effect of drugs. The results of Zhong Mei et al. 21 ‘s study showed that after 0.02% atropine was used once a day for 12 months, the pupil dilation was about 0.84mm, and the decrease in AMP was about 1.12D, which was lower than the results of our study, and the analysis may be related to the length of administration. After 0.02% of atropine administration frequency was changed to 2 times a day for 1 week, PD increased by 1.69mm and AMP decreased by 4.53D, suggesting that PD had no obviously cumulative effect, but AMP reduction showed a cumulative effect with continuous duration and frequency of medication. These results suggest that more attention should be paid to AMP compared with PD in the clinical application of low concentration atropine.
The results of this study showed that in the 0.04% atropine group, the pupil dilation was about 2.75 mm larger and the AMP was about 3.78 D lower than the baseline value after a single use. After 1 week of continuous use, the pupil dilated by 1.95mm and AMP decreased by 5.12D. After 2 weeks of continuous use, the pupil dilated by 1.95mm and AMP decreased by 4.66D, which was the similar as the decrease of AMP after 2 weeks of 0.02% atropine group. In 0.04% atropine group, the pupil dilation significantly decreased with the extension of administration time, and AMP decreased by up to 35% at 2 weeks after administration. In the clinical use of atropine, more attention should be paid to the changes in AMP before and after treatment to avoid the near vision blur, binocular vision dysfunction and asthenopia caused by the severe decline in AMP. In LAMP study, after 2 years of follow-up, the mean pupil dilation of 0.01%, 0.025% and 0.05% atropine groups were 0.60mm, 0.67mm and 1.25 mm, respectively, and AMP decreased by 0.63 D, 1.66 D and 2.05 D 12, respectively. In this study, PD changes and AMP declines were more obvious than those in LAMP study, which may be related to different subjects, drug preparation methods, detection methods and medication periods, as well as different detection time points. In this study, the examination time was set from 8:00 AM to 11:00 AM, which was considered to be the most obvious time period after medication.
The questionnaire revealed that subjects in the three groups showed different degrees of photophobia, dryness, asthenopia and other symptoms during medication. One week after medication, OSDI score increased compared with that before medication, and two weeks after medication, OSDI score decreased to the level before medication. There was no statistically significant difference in overall OSDI score among the three groups. It may be related to eye discomfort or tension after the patient’s initial use of the drug. Compared with 0.01% atropine group and 0.02% atropine group, subjects in 0.04% atropine group had more obvious photophobia, which could be relieved by wearing a hat. 4 subjects in 0.04% atropine group had near-vision blur during daily eye use, and needed to place the reading objects at a longer distance to see clearly. There were 4, 4 and 8 patients with mild dryness in 0.01% atropine, 0.02% atropine and 0.04% atropine groups, respectively, suggesting that increasing atropine concentration may affect patients’ subjective feelings, but there were no significant differences in NIBUTfirst, NIBUTaverage and TMH among the three groups. This was consistent with the results of Cheng et al. 22, which found that there were no statistically significant differences in NIBUTfirst, NIBUTaverage and TMH after 0.01% atropine treatment for 6 months compared with that before treatment. After 2 weeks of medication, NIBUTfirst, NIBUTaverage and TMH of subjects in the 3 groups were increased to varying degrees compared with before medication. It was considered that the possible reason was long-term eye stimulation after continuous medication, which improved tear secretion and tear film quality.
In summary, after the use of atropine at different concentrations, PD dilated and AMP decreased in different degrees, and the higher the concentration, the more serious it was. Meanwhile, after the frequency of 0.01% and 0.02% atropine was increased, the decrease degree of PD and AMP was more obvious than that while it was used once a night, but no adverse reactions witch cannot be tolerated were found. With 0.04% atropine used for 2 weeks, AMP decreased by about 35%. It was suggested that the clinical use of atropine eye drops should gradually increase the drug concentration from a low concentration (0.01%, 0.02%), or increase the frequency of atropine use, so as to increase the tolerance and adaptability of patients. In view of the small sample size included in this study and the short observation time, it is necessary to further expand the sample size and prolong the observation period in the future to verify the conclusion.
Conflict of Interest All authors declare that there is no conflict of interest
Author Contribution Statement He Meinan: Conception and design of experiments, conducting study, data collection/ analysis/interpretation, statistical analysis, drafting; Mi Baoyue: Conducting study, data collection/analysis/interpretation, statistical analysis, drafting; Zhu Ying: Data analysis/interpretation, article’s intellectual content review, conducting study; Liu Lin: Conception and design of experiments, article’s intellectual content review/guidance; Zhang Ziyu: Conducting study, data collection, statistical analysis, drafting; Du Bei: Conception and design of experiments, article’s intellectual content review/guidance; Wei Ruihua: Conception and design of experiments, guidance, article’s intellectual content review/finalizing
References
[1] Morgan IG, French AN, Ashby RS, et al. The epidemics of myopia: aetiology and prevention[J]. Prog Retin Eye Res, 2018, 62:134-149. DOI: 10.1016/j.preteyeres.2017.09.004.
[2] Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050[J]. Ophthalmology, 2016, 123(5):1036-1042. DOI: 10.1016/j.ophtha.2016.01.006.
[3] Yokoi T, Ohno-Matsui K. Diagnosis and treatment of myopic maculopathy[J]. Asia Pac J Ophthalmol (Phila), 2018, 7(6):415-421. DOI: 10.22608/APO.2018290.
[4] Chang L, Pan CW, Ohno-Matsui K, et al. Myopia-related fundus changes in Singapore adults with high myopia[J]. Am J Ophthalmol, 2013, 155(6):991-999. DOI: 10.1016/j.ajo.2013.01.016.
[5] Flitcroft DI. The complex interactions of retinal, optical and environmental factors in myopia aetiology[J]. Prog Retin Eye Res, 2012, 31(6):622-660. DOI: 10.1016/j.preteyeres.2012.06.004.
[6] Brennan NA, Toubouti YM, Cheng X, et al. Efficacy in myopia control[J/OL]. Prog Retin Eye Res, 2021, 83:100923[2022-05-01]. http://www.ncbi.nlm.nih.gov/pubmed/33253901. DOI: 10.1016/j.preteyeres.2020.100923.
[7] Huang J, Wen D, Wang Q, et al. Efficacy comparison of 16 interventions for myopia control in children: a network meta-analysis[J]. Ophthalmology, 2016, 123(4):697-708. DOI: 10.1016/j.ophtha.2015.11.010.
[8] Tran H, Tran YH, Tran TD, et al. A review of myopia control with atropine[J]. J Ocul Pharmacol Ther, 2018, 34(5):374-379. DOI: 10.1089/jop.2017.0144.
[9] Chia A, Chua WH, Cheung YB, et al. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (atropine for the treatment of myopia 2)[J]. Ophthalmology, 2012, 119(2):347-354. DOI: 10.1016/j.ophtha.2011.07.031.
[10] Chia A, Lu QS, Tan D. Five-year clinical trial on atropine for the treatment of myopia 2: myopia control with atropine 0.01% eyedrops[J]. Ophthalmology, 2016, 123(2):391-399. DOI: 10.1016/j.ophtha.2015.07.004.
[11] Wei S, Li SM, An W, et al. Safety and efficacy of low-dose atropine eyedrops for the treatment of myopia progression in Chinese children: a randomized clinical trial[J]. JAMA Ophthalmol, 2020, 138(11):1178-1184. DOI: 10.1001/jamaophthalmol.2020.3820.
[12] Yam JC, Li FF, Zhang X, et al. Two-year clinical trial of the low-concentration atropine for myopia progression (LAMP) study: phase 2 report[J]. Ophthalmology, 2020, 127(7):910-919. DOI: 10.1016/j.ophtha.2019.12.011.
[13] Qin J, Lyu Y, Wei L, et al. Comparison of myopia progression between different concentrations and application frequencies of atropine eye drops in children[J]. Chin J Exp Ophthalmol, 2021, 39(5):423-429. DOI: 10.3760/cma.j.cn115989-20200101-00015.
[14] Chinese Optometri Association, Chinese Ophthalmological Society. Consensus guidelines of refractive correction for children[J]. Chin J Optom Ophthalmol Vis Sci, 2017, 19(12):705-710. DOI: 10.3760/cma.j.issn.1674-845X.2017.12.001.
[15] Lyu Y, Ji N, Fu AC, et al. Comparison of administration of 0.02% atropine and orthokeratology for myopia control[J]. Eye Contact Lens, 2021, 47(2):81-85. DOI: 10.1097/ICL.0000000000000699.
[16] Bullimore MA, Berntsen DA. Low-dose atropine for myopia control: considering all the data[J/OL]. JAMA Ophthalmol, 2018, 136(3):303[2022-05-06]. http://www.ncbi.nlm.nih.gov/pubmed/29423500. DOI: 10.1001/jamaophthalmol.2017.6638.
[17] Yam JC, Jiang Y, Tang SM, et al. Low-concentration atropine for myopia progression (LAMP) study: a randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in myopia control[J]. Ophthalmology, 2019, 126(1):113-124. DOI: 10.1016/j.ophtha.2018.05.029.
[18] He MN, Zhu Y, Wang XL, et al. Effects of 0.1 g·L-1 atropine on pupil size and accommodation[J]. Rec Adv Ophthalmol, 2016, 36(10):932-935. DOI: 10.13389/j.cnki.rao.2016.0249.
[19] Chen Z, Li T, Yao P, et al. Effects of 0.05% racanisodamine on pupil size and accommodation[J]. Optom Vis Sci, 2010, 87(12):966-970. DOI: 10.1097/OPX.0b013e3181fc6445.
[20] Fu A, Stapleton F, Wei L, et al. Effect of low-dose atropine on myopia progression, pupil diameter and accommodative amplitude: low-dose atropine and myopia progression[J]. Br J Ophthalmol, 2020, 104(11):1535-1541. DOI: 10.1136/bjophthalmol-2019-315440.
[21] Zhong M, Lyu Y, Fu AC, et al. Effects of 0.01% and 0.02% atropine eye drops on pupil diameter and accommodation amplitude in myopic children: one-year randomized, double blind, controlled trail[J]. Chin J Exp Ophthalmol, 2019, 37(7):540-545. DOI: 10.3760/cma.j.issn.2095-0160.2019.07.009.
[22] Cheng J, Yang Y, Kong X, et al. The effect of 0.01% atropine eye drops on the ocular surface in children for the control of myopia-the primary results from a six-month prospective study[J]. Ther Clin Risk Manag, 2020, 16:735-740. DOI: 10.2147/TCRM.S265945.