·Experimental Research·
Distribution of coronavirus disease 2019 transmission-related receptors angiotensin-converting enzyme 2 and transmembrane serine protease 2 in human conjunctival tissue and its significance
Liu Hui1, Li Yuanpeng1, Yang Jingru1, Ren Yujie1, Wang Weiwei2, Cai Fengmei1, Xia Yimin1, Wang jia1, Wang Huifang1
1Department of Pathology, Xi’an People’s Hospital, Xi’an Fourth Hospital, Xi’an 710004, China; 2Department of Ophthalmology, Xi’an People’s Hospital, Xi’an Fourth Hospital, Xi’an 710004, China
Corresponding author: Wang Weiwei, Email: 1248038151@qq.com
[Abstract] [View PDF in English] [View PDF in Chinese] [Read Full Text]
Objective To investigate the expression of coronavirus disease 2019 (COVID-19) transmission-related receptors angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) in human conjunctival tissue and its clinical significance.
Methods Fifty human conjunctival tissue specimens from 50 patients including 10 normal conjunctival tissues, 15 conjunctival papilloma tissues, 15 conjunctival nevus tissues and 10 conjunctival cyst tissues were collected from June 2019 to June 2020 at Xi’an People’s Hospital. Ten corneal tissue samples from 10 patients with eyes removed due to trauma were collected as control. The distribution of ACE2 and TMPRSS2 in different corneal tissues was detected by the immunohistochemistry. The expression of ACE2 and TMPRSS2 was scored and compared. Reuse of the human samples and the research protocol was approved by an Ethics Committee of Xi’an People’s Hospital (No.20190022). Written informed consent was obtained from each patient.
Results ACE2 and TMPRSS2 were both expressed in normal conjunctival epithelium, epithelial cells in conjunctiva papilloma and conjunctival nevus, and cells in conjunctiva cyst wall.ACE2 was mainly distributed in the superficial and intermediate cells of conjunctival epithelium, but not in the basal cells and goblet cells.TMPRSS2 was found in different layers of cells. The positive expression rates of ACE2 and TMPRSS2 in conjunctiva were both 100%.There was no significant difference in the expression intensity of ACE2 and TMPRSS2 among normal conjunctival tissue, conjunctival papilloma, conjunctival nevus and conjunctival cyst (all at P>0.05). Weakly expressed in corneal tissues, ACE2 and TMPRSS2 were more moderately and strongly expressed in conjunctival tissues. There were significant differences in the number of differently graded ACE2 and TMPRSS2 expression between normal conjunctival tissues, conjunctival papilloma, conjunctival nevus, conjunctival cyst and corneal tissues (ACE2: Z=-3.473, -4.183, -3.970, -3.873, all at P<0.01; TMPRSS2: Z=-4.119, -4.472, -4.443, -4.147, all at P<0.001).
Conclusions COVID-19 transmission-related receptors ACE2 and TMPRSS2 are expressed in human conjunctival tissue, which provides organological evidence for ocular surface transmission of COVID-19.
[Key words] COVID-19; Conjunctiva; Angiotensin-converting enzyme 2; Transmembrane serine protease 2
Fund program: Research and Incubation Fund Project of Xi’an People’s Hospital (FZ-38)
DOI: 10.3760/cma.j.cn115989-20210126-00065
Coronavirus disease 2019 (COVID-19) is mainly transmitted through droplet, close contact, or aerosol, causing respiratory tract infection and pneumonia in humans, which can rapidly progress to acute respiratory distress syndrome, septicemic shock, and multi-organ failure. The International Committee on Taxonomy of Viruses announced the English name of the COVID-19 virus as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Studies have shown that SARS-CoV-2, similar to SARS-CoV, has a high affinity for the angiotensin-converting enzyme 2 (ACE2) receptor on host cells, targets the ACE2 receptor on the host cell using the envelope spinosin (S protein), and is further activated by transmembrane serine protease 2 (TMPRSS2), which mediates viral entry into the host cell, thus playing an important role in the process of COVID-19 infection 1-3. Ocular surface tissues, such as the conjunctiva and cornea, are directly exposed to the outside world, and more experimental data are needed to investigate whether SARS-CoV-2 can infect humans through the ocular surface. In this study, we investigated the expression of ACE2 and TMPRSS2 in human ocular conjunctival tissues to provide insight into whether SARS-CoV-2 can infect humans through ocular surface tissues.
1 Materials and Methods
1.1 Materials
1.1.1 Source and grouping of tissue specimens
Fifty human conjunctival tissue specimens (10 cases of normal conjunctival tissues, 15 cases of conjunctival papilloma tissues, 15 cases of conjunctival nevus tissues, and 10 cases of conjunctival cyst tissues) excised from the Department of Ophthalmology of Xi’an People’s Hospital from June 2019 to June 2020 were collected as the case group, with an age range of 23–75 years and mean of 48.3 years. Ten corneal tissues were collected as the control group, aged 28–61 years, with a mean of 44.7 years. The normal conjunctiva for this study was obtained from the normal conjunctival tissue surrounding benign conjunctival lesions, and the corneal tissue was obtained from the eyes with well-preserved corneal tissue that had been removed due to trauma. The study protocol was approved by the Ethics Committee of Xi’an People’s Hospital (No. 20190022), and informed consent was obtained from patients for the use of pathological specimens. All pathological sections were reread by 2 experienced pathologists, and clinical information was collected for all cases.
1.1.2 Main reagents and instruments
The main reagents and instruments used included the following: rabbit monoclonal ACE2 antibody (ab108252), rabbit monoclonal TMPRSS2 antibody (ab109131) (Abcam, USA), LEICA immunohistochemical development kit (100203040113) (Leica Biosystems GmbH, Germany), and LEICA BOND-MAX fully automated immunohistochemistry instrument (Leica Biosystems GmbH, Germany).
1.2 Methods
1.2.1 Determination of the ACE2 and TMPRSS2 expression based on immunohistochemistry
All tissues were fixed with neutral formaldehyde at a mass fraction of 10%, paraffin-embedded, and serially sectioned at a thickness of 4 μm. The tissues were stained using the EnVision method, and a LEICA BOND-MAX automatic immunohistochemistry machine was used. The specific procedures were performed according to the manufacturer’s instructions. Kidney tissue was used as a positive control for ACE2, and prostate cancer tissue was used as a positive control for TMPRSS2. The negative controls were treated with phosphate buffer instead of the primary antibody. The dilution ratio of ACE2 antibody was 1:200, and that of TMPRSS2 antibody was 1:1,000.
1.2.2 Interpretation of immunohistochemical results
The ACE2- and TMPRSS2-positive results were defined as ACE2- and TMPRSS2-positive staining localized to the cell pulp and cell membrane with brownish-yellow granules. Scoring was based on the degree of staining and percentage of positive cells: 0 for unstained, 1 for yellow, 2 for brown, and 3 for tan—0 for the percentage of positive cells <10%, 1 for 10%–25%, 2 for 26%–50%, 3 for 51%–75%, and 4 for >75%. Each section was divided into four levels according to the score obtained by multiplying its staining score and percentage of positive cells: 0 as negative (−), 1–4 as weakly positive (+), 5–8 as moderately positive (++), and 9–12 as strongly positive (++++) 4.
1.3 Statistical methods
SPSS 22.0 was used for statistical analysis. The count data were expressed as frequencies, and the Mann–Whitney U test was used for the difference comparison of the cases in different intensities of ACE2 expression between different tissues, Statistical significance was set at P < 0.05.
2 Results
2.1 Expression of ACE2 in human conjunctival tissues
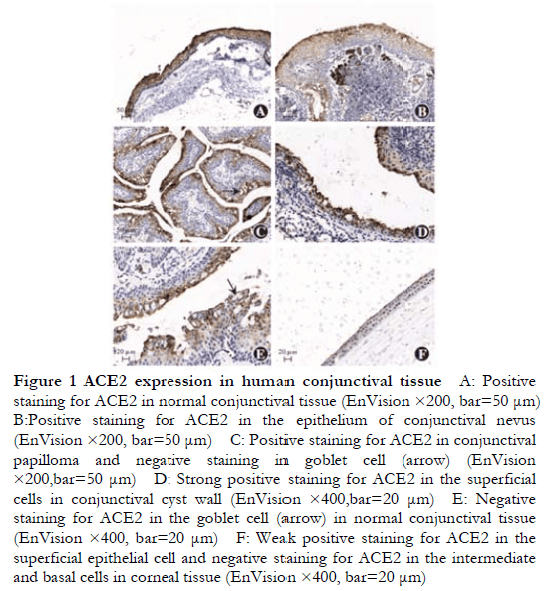
ACE2 showed a moderate-to-strong positive expression (++ to +++) in 50 conjunctival tissues of the test group, with a positive expression rate of 100%, mainly localized in the cell membrane and cytoplasm, expressed in the normal conjunctival epithelium, epithelial cells in the conjunctival papilloma and conjunctival nevus, and cells in the conjunctival cyst wall (Figure 1A–D). ACE2 was expressed in the superficial and intermediate cells of the conjunctival epithelium but not in the basal and goblet cells (Figure 1E). There was no significant difference in expression among the normal conjunctival tissue, conjunctival papilloma, conjunctival nevus, and conjunctival cyst. In the control group, ACE2 was expressed only in normal superficial corneal cells, and no expression was observed in basal lamina cells (Figure 1F).
2.2 Expression of TMPRSS2 in human conjunctival tissues
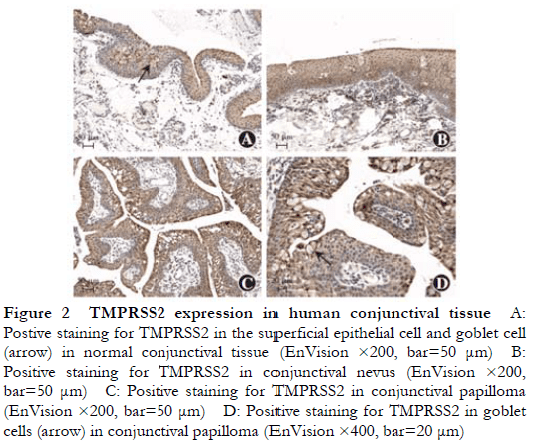
TMPRSS2 showed a weak-to-strong positive expression (+ to ++++) in the conjunctival tissues of 50 cases in the experimental group, with a positive expression rate of 100%, mainly localized in the cytoplasm and expressed in the conjunctival epithelium, epithelial cells in the conjunctival papilloma and conjunctival nevus, and cells in the conjunctival cyst wall. TMPRSS2 was expressed throughout the conjunctival epithelium and in the goblet cells (Figure 2), and there were no significant differences in expression among the normal conjunctival tissues, conjunctival papillomas, conjunctival nevi, and conjunctival cysts.
2.3 Comparison of the ACE2 and TMPRSS2 expression in conjunctival and corneal tissues of different patients
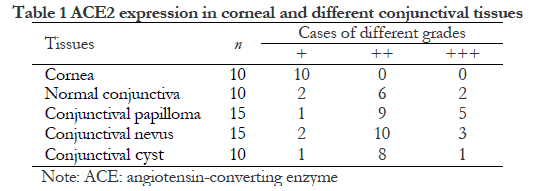
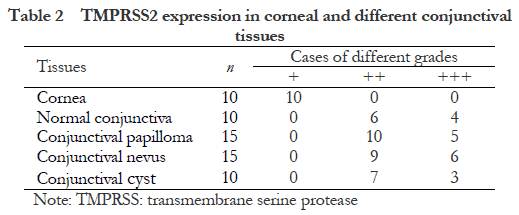
None of the 60 patients had a history of SARS-CoV-2 infection. The differences in the expression intensity of ACE2 and TMPRSS2 in the different conjunctival tissues were not statistically significant (all P > 0.05). ACE2 and TMPRSS2 showed a weak positive expression in the corneal tissues. Compared with corneal tissues, there were more cases of moderate positive and strong positive expression of ACE2 and TMPRSS2 in the normal conjunctival tissues, conjunctival papillomas, conjunctival nevi, and conjunctival cysts, all with statistically significant differences (ACE2, Z = −3.473, −4.183, −3.790, and −3.873, all P < 0.01; TMPRSS2, Z = −4.119, −4.472, −4.443, and −4.147, all P < 0.001) (Tables 1 and 2).
3 Discussion
SARS-CoV-2 is mainly transmitted throughby droplets, close contact, or aerosols. Whether the ocular surface in direct contact with air can serve as a transmission route for COVID-19 is controversial. Most previous studies have focused on animal models. Deng et al. 5 showed positive conjunctival swab results in rhesus monkeys 1 day after conjunctival inoculation with SARS-CoV-2, and their nasal and pharyngeal swab results were positive from 1–7 days. This indicates that SARS-CoV-2 can infect rhesus monkeys through the conjunctival route and can be transferred from the conjunctiva to tissues, such as the nasal cavity and respiratory tract. Xia et al. 6 detected SARS-CoV-2 in tear and conjunctival secretions of patients with COVID-19. Many studies have also shown that patients with COVID-19 may present with conjunctival symptoms as their initial symptoms. In a study of 535 patients with COVID-19, Chen et al. 9 found 27 patients with conjunctival congestion, 4 with conjunctival congestion as the initial symptom, and 332 with a history of hand–eye contact. They found that hand–eye contact in patients with COVID-19 was independently associated with conjunctival congestion. They also found that some patients with COVID-19 had chronic eye diseases, including conjunctivitis, dry eye, and keratitis. Wu et al. 10 studied 38 patients with COVID-19 and found that 12 of them had conjunctivitis. Among the 12 patients with ocular symptoms, 2 had positive conjunctival swab results. This study suggests that SARS-CoV-2 is transmitted to humans through the ocular surface.
However, some researchers have questioned whether SARS-CoV-2 can infect humans through the ocular surface. Liang et al. 11 analyzed conjunctival swab samples from 37 patients with COVID-19, and 3 of the 37 patients had symptoms of conjunctivitis. In one patient without conjunctivitis, SARS-CoV-2 was detected in conjunctival swab samples. Peng et al. 12 attributed conjunctivitis to a “coincidental event” and concluded that there was no causal relationship between SARS-CoV-2 infection and conjunctivitis. In light of these controversies, Aiello et al. 13 retrospectively analyzed the literature on SARS-CoV-2 and ocular tissues and secretions and found that 3 patients with conjunctivitis tested positive for SARS-CoV-2 in tears, 14 patients with conjunctivitis tested negative for SARS-CoV-2 in tears, and 8 patients tested positive for SARS-CoV-2 in tears without conjunctivitis. The authors concluded that although most of the studies were controversial, we cannot exclude the possibility of COVID-19 as a secondary route of transmission through ocular structures because conjunctival secretions and tear fluid tested positive for SARS-CoV-2 in these patients.
ACE2 and TMPRSS2 play important roles in SARS-CoV-2 infection. SARS-CoV-2 binds to ACE2 receptors on host cells using S proteins and is further activated by cleavage via host cell TMPRSS2 to mediate viral entry into host cells. However, there are controversies regarding the existence of the ACE2 and TMPRSS2 expression in ocular surface tissues and whether SARS-CoV-2 mediates infection in humans through ACE2 and TMPRSS2 in ocular surface tissues. Zhou et al. 14 examined the expression of ACE2 and TMPRSS2 in human tissues using immunohistochemistry and western blotting and found that both were constantly expressed in the conjunctival and corneal epithelial cells but not in the goblet cells, suggesting that SARS-CoV-2 infects humans through the conjunctival epithelial cells. Collin et al. 15 also found a positive expression of ACE2 and TMPRSS2 in the adult conjunctiva, cornea, and corneal rim, suggesting that ocular surface epithelial tissues provide a new route for SARS-CoV-2 infection in humans. In this study, 50 human conjunctival tissues and 10 normal corneal tissues were examined by immunohistochemistry, and different levels of the ACE2 and TMPRSS2 expression were found, which is consistent with the results of previous studies. In addition, this study found no significant difference in the expression of ACE2 and TMPRSS2 among the normal conjunctiva, conjunctival nevi, conjunctival papillomas, and conjunctival cysts, suggesting the possibility of SARS-CoV-2 infection. However, other studies have also reported contradictory results. Lange et al. 16 examined the expression of ACE2 and TMPRSS2 in 38 conjunctival tissues using RNA sequencing and immunohistochemistry and did not find ACE2 expression in normal or abnormal conjunctival tissues, consistent with the results obtained by Xiang et al. 17. Ma et al. 18 examined the expression of ACE2 and TMPRSS2 in the human conjunctiva, pterygium cell lines, and murine corneal tissues using reverse transcription polymerase chain reaction and showed that conjunctival cells were less likely to be infected by SARS-CoV-2, whereas pterygium cell lines were more likely to be infected by SARS-CoV-2. A high and constant ACE2 and TMPRSS2 expression was observed in murine corneal tissues, suggesting that SARS-CoV-2 is more likely to infect humans through corneal tissues. Although most current studies support the conclusion of the presence of the ACE2 and TMPRSS2 expression in human conjunctival tissues, a few studies have yielded inconsistent results, which may be related to the experimental methods used, source of the tissues, and quality of the antibodies. Further studies are required to confirm this hypothesis.
Summarizing the results of this study and previous literature, most researchers believe that ocular protection is essential to prevent coronavirus infection, especially when healthcare workers are treating patients with COVID-19 in close proximity. The presence of SARS-CoV-2 in patients’ tears and ocular secretions cannot be ruled out, and tear and conjunctival secretions may increase the chances of the virus entering the body through the nasolacrimal duct and causing infection 21, thus necessitating goggles to prevent infection through ocular contact 19,22. A limitation of this study is that we did not obtain ocular surface tissues from patients with COVID-19; therefore, the expression of ACE2 and TMPRSS2 in the ocular surface tissues of the patients involved could not be obtained. Although controversy remains, most studies have indicated the presence of the ACE2 and TMPRSS2 expression in human conjunctival tissues. Whether SARS-CoV-2 can be transmitted through the conjunctiva as a route of transmission needs to be further investigated, as this route may increase the risk of patients developing systemic SARS-CoV-2 infection.
Conflict of interest None declared
Author contributions Liu H: Study design and implementation, data collection and analysis, manuscript writing; Li YP, Ren YJ: Experiment operation, data analysis; Yang JR, Wang WW, Xia YM, Wang J: Experiment design, data collection; Cai FM. Wang HF: Study design, manuscript review
References
[1] Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin[J]. Nature, 2020, 579(7798):270-273. DOI: 10.1038/s41586-020-2012-7.
[2] Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor[J]. Cell, 2020, 181(2):271-280. DOI: 10.1016/j.cell.2020.02.052.
[3] Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation[J]. Science, 2020, 367(6483):1260-1263. DOI: 10.1126/science.abb2507.
[4] Huo Z, Xuan X, Wu SF, et al. Diagnostic value of MYB protein expression in adenoid cystic carcinoma and status of MYB gene copy number[J]. Chin J Pathol, 2015, 44(8):582-586. DOI: 10.3760/cma.j.issn.0529-5807.2015.08.008.
[5] Deng W, Bao L, Gao H, et al. Rhesus macaques can be effectively infected with SARS-CoV-2 via ocular conjunctival route[J/OL]. bioRxiv, 2020[2021-12-05]. https://www.biorxiv.org/content/10.1101/2020.03.13.990036v1.
[6] Xia J, Tong J, Liu M, et al. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection[J]. J Med Virol, 2020, 92(6):589-594. DOI: 10.1002/jmv.25725.
[7] Ye Y, Song YP, Yan M, et al. Novel coronavirus pneumonia combined with conjunctivitis: three cases report[J]. Chin J Exp Ophthalmol, 2020, 38(3):242-244. DOI: 10.3760/cma.j.issn.2095-0160.2020.0006.
[8] Li XJ, Wang M, Chen CZ, et al. Ophthalmologists’ strategy for the prevention and control of coronavirus pneumonia with conjunctivitis or with conjunctivitis as the first symptom[J]. Chin J Exp Ophthalmol, 2020, 38(3):276-280. DOI: 10.3760/cma.j.issn.2095-0160.2020.0002.
[9] Chen L, Deng C, Chen X, et al. Ocular manifestations and clinical characteristics of 535 cases of COVID-19 in Wuhan, China: a cross-sectional study[J/OL]. Acta Ophthalmol, 2020, 98(8):e951-e959[2021-12-05]. https://pubmed.ncbi.nlm.nih. gov/32421258/. DOI: 10.1111/aos.14472.
[10] Wu P, Duan F, Luo C, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei province, China[J]. JAMA Ophthalmol, 2020, 138(5):575-578. DOI: 10.1001/jamaophthalmol.2020.1291.
[11] Liang L, Wu P. There may be virus in conjunctival secretion of patients with COVID-19[J/OL]. Acta Ophthalmol, 2020, 98(3):223[2021-12-07]. https://pubmed.ncbi.nlm.nih.gov/32189460/. DOI: 10.1111/aos.14413.
[12] Peng Y, Zhou YH. Is novel coronavirus disease (COVID-19) transmitted through conjunctiva?[J]. J Med Virol, 2020, 92(9):1408-1409. DOI: 10.1002/jmv.25753.
[13] Aiello F, Gallo Afflitto G, Mancino R, et al. Coronavirus disease 2019 (SARS-CoV-2) and colonization of ocular tissues and secretions: a systematic review[J]. Eye (Lond), 2020, 34(7):1206-1211. DOI: 10.1038/s41433-020-0926-9.
[14] Zhou L, Xu Z, Castiglione GM, et al. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection[J]. Ocul Surf, 2020, 18(4):537-544. DOI: 10.1016/j.jtos.2020.06.007.
[15] Collin J, Queen R, Zerti D, et al. Co-expression of SARS-CoV-2 entry genes in the superficial adult human conjunctival, limbal and corneal epithelium suggests an additional route of entry via the ocular surface[J]. Ocul Surf, 2021, 19:190-200. DOI: 10.1016/j.jtos.2020.05.013.
[16] Lange C, Wolf J, Auw-Haedrich C, et al. Expression of the COVID-19 receptor ACE2 in the human conjunctiva[J]. J Med Virol, 2020, 92(10):2081-2086. DOI: 10.1002/jmv.25981.
[17] Xiang M, Zhang W, Wen H, et al. Comparative transcriptome analysis of human conjunctiva between normal and conjunctivochalasis persons by RNA sequencing[J]. Exp Eye Res, 2019, 184:38-47. DOI: 10.1016/j.exer.2019.04.005.
[18] Ma D, Chen CB, Jhanji V, et al. Expression of SARS-CoV-2 receptor ACE2 and TMPRSS2 in human primary conjunctival and pterygium cell lines and in mouse cornea[J]. Eye (Lond), 2020, 34(7):1212-1219. DOI: 10.1038/s41433-020-0939-4.
[19] Li JO, Lam D, Chen Y, et al. Novel coronavirus disease 2019 (COVID-19): The importance of recognising possible early ocular manifestation and using protective eyewear[J]. Br J Ophthalmol, 2020, 104(3):297-298. DOI: 10.1136/bjophthalmol-2020-315994.
[20] Lai T, Tang E, Chau S, et al. Stepping up infection control measures in ophthalmology during the novel coronavirus outbreak: an experience from Hong Kong[J]. Graefes Arch Clin Exp Ophthalmol, 2020, 258(5):1049-1055. DOI: 10.1007/s00417-020-04641-8.
[21] Qing H, Li Z, Yang Z, et al. The possibility of COVID-19 transmission from eye to nose[J/OL]. Acta Ophthalmol, 2020, 98(3):e388[2021-12-08]. https://pubmed.ncbi.nlm.nih.gov/32189463/. DOI: 10.1111/aos.14412.
[22] Lu CW, Liu XF, Jia ZF. 2019-nCoV transmission through the ocular surface must not be ignored[J/OL]. Lancet, 2020, 395(10224):e39[2021-12-08]. https://pubmed.ncbi.nlm.nih.gov/32035510/. DOI: 10.1016/S0140-6736(20)30313-5.