•Clinical Research•
Effect and safety of aflibercept in the treatment of polypoidal choroidal vasculopathy with ranibizumab-resistant serous pigment epithelial detachment
Zhou Pengyi, Yang Lin, Xu Youmei, Pan Meng, Guo Ju, Du Liping, Jin Xuemin
Department of Ophthalmology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, China
Corresponding author: Jin Xuemin, Email: jinxuemin@zzu.edu.cn
[Abstract] [View PDF in English] [View PDF in Chinese] [Read Full Text]
Objective To evaluate the effectiveness and safety of intravitreal injection of different doses of aflibercept for polypoidal choroidal vasculopathy (PCV) with serous pigment epithelial detachment (PED) resistant to ranibizumab.
Methods A non-randomized controlled clinical study was conducted. Seventy-three eyes of 73 patients with PCV and serous PED resistant to ranibizumab were enrolled at the First Affiliated Hospital of Zhengzhou University from January 2019 to December 2020. All patients were treated by intravitreal injection of 2 mg or 4 mg aflibercept according to the patient’s willingness: 2 mg aflibercept or 4 mg aflibercept was intravitreally injected monthly for three consecutive months following a pro re nata (PRN) regimen in the 2 mg aflibercept group (38 eyes) and 4 mg aflibercept group (35 eyes), respectively. PED height and central macular thickness (CMT) were measured by optical coherence tomography, and the best corrected visual acuity (BCVA) was assessed with a visual acuity chart and converted to logarithm of the minimum angle of resolution (LogMAR) units before the injection and at 1, 2, 3, and 6 months after the first injection. Intraocular pressure and treatment-related adverse events were recorded. This study adhered to the Declaration of Helsinki and was approved by an Ethics Committee of The First Affiliated Hospital of Zhengzhou University (No. 2021-KY-1252). Written informed consent was obtained from each patient prior to entering the study cohort.
Results Thirty-three patients (86.84%) in the 2 mg aflibercept group and 30 patients (85.71%) in the 4 mg aflibercept group finished the treatment and follow-up, respectively. The PED, BCVA, and CMT before treatment and at the end of follow-up were respectively 379.24±95.50 µm and 280.09±120.50 µm, 0.68±0.27 µm and 0.51±0.19 µm, and 393.96±100.81 and 291.70±44.09 µm in the 2 mg aflibercept group, showing statistically significant differences (all P<0.05). The PED, BCVA, and CMT before treatment and at the end of follow-up were respectively 393.07±93.76 and 278.63±145.07 µm, 0.66±0.31 µm and 0.48±0.22 µm, and 377.43±79.61 µm and 284.67±84.88 µm in the 4 mg aflibercept group, with statistically significant differences (all P<0.05). The CMT value in the 4 mg aflibercept group was significantly lower than that in the 2 mg aflibercept group at 1 month after injection (P<0.05). No severe ocular and systemic adverse events were found during the follow-up, such as retinal detachment, endophthalmitis, cataract, and persistent high intraocular pressure.
Conclusions Both 2 mg and 4 mg aflibercept can effectively treat ranibizumab-resistant PCV with serous PED, and improve the anatomical structure of the retina
and BCVA. In addition, 4 mg aflibercept can accelerate the recovery of PED and CMT.
[Key words] Polyps; Choroidal neovascularization; Pigment epithelial detachment, serous; Introvitreal injection; Recombinant fusion proteins/therapeutic use; Treatment outcome; Visual acuity
Fund program: National Natural Science Foundation of China (81800832, 81970792, 8217040), Medical Science and Technology Research Program of Henan Province (201602081)
DOI: 10.3760/cma.j.cnll5989-20211206-00671
Polypoidal choroidal vasculopathy (PCV) is a vascular disease characterized by choroidal vascular polypoid lesions with or without abnormal branch vascular networks, which is often accompanied by recurrent serous or hemorrhagic retinal pigment epithelial detachment (PED). PCV is one of the main causes of vision loss or blindness in the elderly [1]. In the aqueous humor of eyes with PCV, vascular endothelial growth factor (VEGF) is highly expressed, which may induce increased vascular permeability and vascular leakage [1]. The PLANET study and the EVEREST II study showed that the visual function and anatomical outcome of patients with PCV could be significantly improved by intravitreal injection of anti-VEGF agents [2-3]. The occurrence of PED is associated with choroidal neovascularization (CNV) and it can develop to a disciform scar, which may result in further impairment of vision [4]. Therapeutic drugs for PCV include monoclonal antibodies and fusion proteins. Among them, fusion protein drugs have the characteristics of combining multiple therapeutic targets, long-term intraocular action time, and high efficiency; they inhibit the division and proliferation of vascular endothelial cells and reduce vascular permeability in turn, achieving the effect of treating neovascular ophthalmopathy [1]. As a fusion protein anti-VEGF drug, aflibercept can effectively treat PCV, with a certain effect on patients with PCV and PED [4]. In China, PED is listed as an overactive lesion that requires intravitreal injection of anti-VEGF drugs, with expert consensus on the intravitreal injection of aflibercept in the treatment of neovascular age-related macular degeneration (AMD) [5]. Aflibercept has been found to improve the best-corrected visual acuity (BCVA) and macular retinal thickness in patients with AMD or PCV resistant to ranibizumab [4]. However, there are few reports on the appropriate dose of aflibercept and its efficacy in patients with ranibizumab-resistant PCV and serous PED. This study was intended to evaluate the effectiveness and safety of intravitreal injection of different doses of aflibercept for eyes with PCV and serous PE0D resistant to ranibizumab.
1 Materials and Methods
1.1 General information
A non-randomized controlled clinical study was conducted to include 73 eyes (39 right eyes and 34 left eyes) of 73 patients who were diagnosed with PCV with serous PED at the First Affiliated Hospital of Zhengzhou University from January 2019 to December 2020. Patients were divided into a 2 mg aflibercept group and a 4 mg aflibercept group in accordance with their wishes, and underwent intravitreal injection with the corresponding doses of aflibercept. There were 38 eyes (20 right eyes and 18 left eyes) of 38 patients in the 2 mg aflibercept group, and 35 eyes (19 right eyes and 16 left eyes) in the 4 mg aflibercept group. All patients with a disease course from 4 to 8 months had received three or more ranibizumab treatments, of which the 2 mg aflibercept group had injected ranibizumab 3–6 times, with an average of 3.9±1.1 times. In the 4 mg aflibercept group, ranibizumab had been injected 3-7 times, with an average of 3.8±1.1 times. Inclusion criteria: (1) Patients aged > 50 years; (2) Patients who met the diagnostic criteria for PCV [6]; (3) Patients with active CNV and serous PED, and PED height > 100 µm; (4) Patients whose optical coherence tomography (OCT) images showed no releasing retinal edema or subretinal fluid compared with the initial treatment, or central macular thickness (CMT) decreased by < 10% compared with before treatment, PED height decreased by < 10% compared with before treatment after ranibizumab administration three consecutive times and at least once a month; (6) Patients who had no injection of an intravitreal drug in the past 1 month [7-8]. Exclusion criteria: (1) Patients with retinal vein occlusion, diabetic retinopathy or other diseases that can induce macular edema or visual changes; (2) Patients with refractive media opacification that could affect the observations; (3) Patients with a medical history of intraocular surgery within 3 months before treatment; (4) Patients who had received retinal laser photocoagulation within 6 months before treatment; (5) Patients who had received photodynamic therapy; (6) Patients who had histories of administration of intravitreal injection of aflibercept, conbercept, triamcinolone acetonide or other glucocorticoids; (7) Patients with intraocular pressure higher than 21 mmHg (1 mmHg = 0.133 kPa) at the first visit; (8) Patients who could not cooperate with the treatment or had follow-up time < 6 months after the first aflibercept injection. There were no significant differences in BCVA, CMT, high PED and other baseline levels between the two groups (all P>0.05) (Table 1). This study adhered to the Declaration of Helsinki and was approved by an Ethics Committee of the First Affiliated Hospital of Zhengzhou University (Approval No.: 2021-KY-1252). All patients voluntarily participated in the study and signed an informed consent form

1.2 Method
1.2.1 Intravitreal injection of aflibercept Levofloxacin eye drops were instilled (1 drop four times a day) before intravitreal injection of aflibercept. Based on the requirements of intraocular surgery, the principles of aseptic treatment were strictly adhered to, and 2 mg (0.05 ml) or 4 mg (0.1 ml) of aflibercept was drawn into a 1 ml syringe. Following puncture, the drug was injected into the pars plana of the superior temporal ciliary body at 3.5 mm to 4.0 mm posterior to the limbus. After injection, the injection sites were compressed by sterile cotton swabs to cover the treated eyes. Slit-lamp microscopy and intraocular pressure measurement were performed at 1 h and 1 day after injection. From the next day, levofloxacin eye drops were instilled into the eyes 4 times a day for 7 days [9]. All eyes were treated with a 3 + pro re nata (PRN) regimen once a month. After 3 months of continuous injection, it was decided whether re-treatment was required according to the follow-up examination results. The criteria for on-demand treatment were established according to the method specified in Reference [4]: (1) The logarithm of the minimum angle of resolution (LogMAR) decreased by ≥ 1 line; (2) Macular retinal edema; (3) Subretinal fluid; and (4) Aggravated PED.
1.2.2 OCT examination of retina Before injection and every month after injection, the CMT of the treated eyes was measured by scanning source optical coherence tomography (SS-OCT) [VG-200D version, Vision Micro Image (Henan) Technology Co., Ltd.]. A 6 mm × 6 mm scan was performed centered on the macula and centered on the maximum height of the PED. The CMT refers to the vertical distance between the inner limiting membrane at the fovea and the outer edge of the retinal pigment epithelium (RPE), and the PED height refers to the maximum height of the PED in the scanned area, which was obtained by manual measurement with the built-in measurement tool of the software.
1.2.3 Follow-up and evaluation indicators Regular follow-up and ophthalmic examination of the treated eyes were performed 1 month after the first, second, and third vitreous injections, respectively, and the follow-up period was 6 months. The results at each time point were defined as the data at 1, 2, 3 and 6 months after the first injection, which were recorded before injection, and at 1, 2, 3 and 6 months after injection. BCVA, PED, and CMT measurements were compared between the two groups before injection and at 1, 2, 3, and 6 months after the first injection in the treated eye; BCVA was converted to logarithm of the minimum angle of resolution (LogMAR) visual acuity and measurements were assessed by the same experienced physician.
1.2.4 Observation of adverse reactions The incidence of endophthalmitis, subconjunctival hemorrhage, RPE tear, ocular hypertension, and other complications was recorded during the follow-up period. The intraocular pressure was monitored 1 h after intravitreal injection. If the intraocular pressure increased, ocular hypotensive eye drops or intravenous mannitol were given to reduce the intraocular pressure.
1.3 Statistical Methods
Statistical analysis was performed using SPSS 22.0 statistical software. It was confirmed by the Shapiro–Wilk test that measurement data for BCVA and PED height and CMT data were in accordance with the normal distribution, and were represented by . The overall difference in each index between the two groups before injection and 1, 2, 3 and 6 months after the first injection were compared by two-way repeated One-way analysis of variance (ANOVA). Moreover, multiple comparisons were performed by least significant difference-t test (LSD-t test). The data for age and number of aflibercept injections in the two groups showed a skewed distribution, expressed as M (Q1, Q3), and the comparisons between groups were conducted by Mann–Whitney U test. Enumeration data were expressed as frequencies, and differences between groups were compared using the χ2 test or the adjusted χ2 test. P<0.05 was considered statistically significant.
2 Results
2.1 Completion of follow-up of participants in the two groups
In the 2 mg aflibercept group, five patients were lost to follow-up, because they could not be followed up in time. Finally, a total of 33 patients completed follow-up, accounting for 86.84%. In the 4 mg aflibercept group, five patients were lost to follow up; they could not be followed up on time owing to the difficulties of follow-up in different places. Finally, a total of 30 patients completed follow-up, accounting for 85.71%. There was no significant difference in age, gender, BCVA, CMT and PED between the two groups (all P>0.05) (Table 2). In the 2 mg aflibercept group, ranibizumab had been injected 3 to 6 times, with an average of 3.8±1.2 times. In the 4 mg aflibercept group, ranibizumab had been injected 3 to 7 times, with an average of 4.0±1.2 times, for which the difference was not statistically significant (t = 0.262, P=0.794).

2.2 Comparison of the timing of aflibercept injections between the two groups
Patients were injected with a total of 3 to 6 injections in the 2 mg aflibercept group, with an average of 4.0 (3.0, 4.5) injections. Patients were injected 3 to 6 times in the 4 mg aflibercept group, with an average of 3.0 (3.0, 4.5) times. There was no significant difference in the number of aflibercept injections between the two groups (Z = –1.097, P=0.273).
2.3 Comparison of PED height before and after injection in the two groups
There was no significant difference in the overall PED height between the two groups (Fgroup=0.455, P=0.502). However, there was a significant difference in the overall PED height between the two groups at different time points before and after injection (Ftime = 14.145, P<0.001), in which the PED height at different time points after injection in each group was significantly lower than that before injection, with statistically significant differences (all P<0.05). The PED height at 2, 3 and 6 months after injection was significantly lower than that at 1 month after injection in the 2 mg aflibercept group, with statistically significant differences (all P<0.05) (Table 3).

2.4 Comparison between the two groups of the proportion of eyes with PED height decreased to less than 50 µm at the last follow-up
At the last follow-up, PED height was decreased to less than 50 in three eyes and six eyes in the 2 mg aflibercept group and 4 mg aflibercept group, accounting for 9.09% and 20.00% respectively, without statistically significant difference (χ2′=0.766, P=0.381); serous PED of two eyes and four eyes was completely healed in the 2 mg aflibercept group and 4 mg aflibercept group respectively, without statistically significant difference (χ2′ = 0.305, P=0.581).
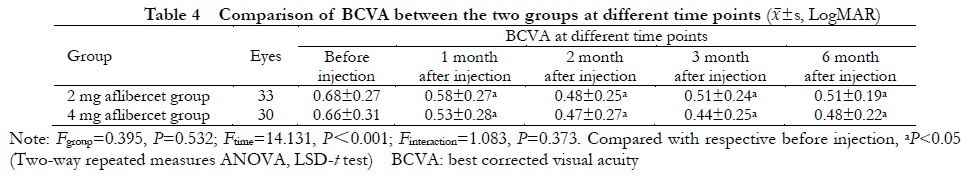
2.5 Comparison of BCVA before and after injection in the eyes of the two groups
There was a significant difference in BCVA between the two groups at different time points before and after injection (Ftime = 14.131, P<0.001): BCVA was increased to various degrees at 1, 2, 3 and 6 months after injection, compared with that before injection, with statistically significant differences (all P<0.05). There was no significant difference in BCVA overall between the two groups (Fgroup=0.395, P=0.532) (Table 4).
 2.6 Comparison of CMT before and after injection between the two groups
2.6 Comparison of CMT before and after injection between the two groups
There was a significant difference in CMT between the two groups at different time points before and after injection (Fgroup=4.272, P=0.043; Ftime=18.747, P<0.001), in which CMT in the 4 mg aflibercept group was lower than that in the 2 mg aflibercept group at 1 month after injection, with statistically significant differences (P<0.05); CMT at different time points after injection was lower than that before injection in each group, with statistically significant differences (all P<0.05); CMT in the 2 mg aflibercept group at 2 months and 3 months after injection was lower than that at 1 month after injection and CMT in the 4 mg aflibercept group at 3 months after injection was lower than that at 2 months after injection, with statistically significant differences (all P<0.05).

2.7 Comparison of the incidence of adverse reactions after treatment between the two groups
In the 2 mg aflibercept group, the intraocular pressure of two eyes increased to 25 mmHg and 27 mmHg 1 h after injection, respectively, which was treated with Brimonidine eye drops and Brimonidine + Timolol eye drops in each eye, respectively. The pressure of the eyes decreased to normal levels after 2 h. Subconjunctival hemorrhage developed in five eyes, which was gradually healed 2 to 4 weeks after injection. The intraocular pressure of four eyes increased to 32 mmHg, 28 mmHg, 27 mmHg, and 27 mmHg 1 h after injection in the 4 mg aflibercept group, which was decreased to normal pressure 2 h after a single instillation of Brimonidine + Timolol eye drops. Subconjunctival hemorrhage developed in six eyes, which gradually disappeared 2 to 4 weeks after injection.
During the follow-up period, no endophthalmitis, vitreous hemorrhage, retinal detachment or other serious ocular and systemic adverse reactions were found in either group.
3 Discussion
PED is common in various chorioretinal diseases, such as neovascular AMD, PCV, and central serous chorioretinopathy [10-11]. Au et al. [12] treated 88 patients with AMD and PED using ranibizumab, aflibercept, and bevacizumab, for which the maximum PED decrease was found in the aflibercept group at 1 month and 12 months of treatment. For patients with ranibizumab-resistant AMD and PED, the visual function and retinal anatomy were improved in some patients after the aflibercept treatment. Broadhead et al. [13] reported that patients in their studies with refractory neovascular AMD were treated with initial loading doses of afliberce for three times. Afterwards, they were treated every 8 weeks. At 48 weeks of treatment, PED was healed, improved, stabilized and worsened in 4 eyes (9.3%), 18 eyes (42%), 24 eyes (56%) and 1 eye (2%), respectively. BCVA was increased by 4.6 letters visible, compared with the condition before treatment. Deng Kaiyu et al. [4] treated 10 patients with PCV and PED using aflibercept 3 + PRN; six patients had more than 25% decrease of PED. In this study, patients with refractory PCV and PED were treated using aflibercept, and it was found that BCVA, CMT, and PED heights were improved to varying degrees after treatment with different doses of aflibercept.
PED is divided into serous, fibrovascular, hemorrhagic, and mixed PED, and anti-VEGF agents are more effective against serous PED than other types of PED [14-15]. Inoue et al. performed intravitreal ranibizumab injections in patients with different types of PED with CNV, based on a 3 + PRN treatment strategy, and found that 100% of patients with serous PED had a decrease in PED height of more than 100 µm during 1 year of follow-up, which was superior to 60.7% of fibrovascular PED and 90.0% of hemorrhagic PED. Rormanek et al. [16] administered intravitreal aflibercept to 36 patients (38 eyes) with PED and CNV. At 12 months of treatment, the average height of PED was decreased by 140 μm. Based on the above study, PCV patients with serous PED were selected as the participants in this study. At the last follow-up, the PED height in the 2 mg aflibercept group and the 4 mg aflibercept group was decreased by an average of 99.15 μm and 114.44 μm compared with those before treatment, respectively. However, there was no significant difference between the two groups. At the last follow-up, PED heights in the eyes of the 4 mg aflibercept group had decreased to less than 50 µm, but were larger than in the 2 mg aflibercept group. There were no significant differences in BCVA between the two groups. However, some studies have shown that there are differences in the therapeutic effects of different doses administered to patients with PCV and PED. You et al. [14] treated 28 patients (33 eyes) with 2 mg aflibercept-tolerant AMD and PED using intravitreal injection of 4 mg aflibercept, and found that macular thickness, subretinal fluid, BCVA and PED were improved to varying degrees in the affected eyes. Chan et al. [17] compared 0.5 mg and 2 mg ranibizumab intravitreal injections in patients with PED. At 1 month after treatment, PED height and decreases in macular retinal thickness were larger in the 2 mg ranibizumab group than in the 0.5 mg ranibizumab group. Moreover, the improvement of BCVA was greater. However, there was no statistically significant difference in each parameter between the two groups at 12 months of follow-up. The results of the study also showed that at 1 month after injection, CMT decreased more in the 4 mg aflibercept group than in the 2 mg aflibercept group, while there was no significant difference in CMT between the two groups at subsequent time points. It was speculated that after the drug dose was increased, the onset time of the drug was faster and more beneficial to the recovery of anatomical structure. However, there was no significant difference in the overall efficacy. Although You et al. [14] obtained beneficial results with high-dose aflibercept in eyes tolerating conventional-dose aflibercept, whether the dose can be increased after the tolerance of conventional-dose aflibercept remains to be further investigated.
Whether there is a correlation between PED and visual acuity is controversial. In the CATT study, there was no statistically significant difference in BCVA between patients with RPE fluid and those patients without RPE fluid at 52 weeks of treatment in 1,142 patients with neovascular AMD [18]. Moreover, at 5 years’ follow-up of 523 patients with neovascular AMD, BCVA in patients with RPE fluid in the macular area (73 letters) was higher than that in patients without RPE fluid (60 letters) as well as in patients with RPE fluid beyond the macular area (60 letters), with statistically significant differences (P=0.006, 0.010) [19]. Similarly, no significant correlations between changes in height, width, and length of PED and BCVA before and after treatment in patients with AMD and PED were found in studies by Broadhead et al. [13]. Cheong et al. [11] studied AMD or PCV eyes with PED and found that the visual prognosis was associated with pretreatment of visual acuity. However, it was not significantly associated with the presence or absence of PED. It has also been shown that long-term PED reactivates the RPE–CNV complex, which could result in increased exudate under the RPE, which could cause a further decrease of visual acuity in turn. When PED is shown to be associated with intraretinal cysts and subretinal fluid, this has a significant effect on BCVA levels at baseline and during follow-up [20]. Therefore, patients with pure PED without subretinal fluid and retinal edema alone were not included in the study. Furthermore, those patients without subretinal fluid, retinal edema and active CNV after intravitreal injection were also excluded.
This study demonstrated that the treatment chosen of 2 mg or 4 mg aflibercept still effectively improved visual acuity and retinal anatomy of patients with ranibizumab-resistant PCV and serous PED. High-dose aflibercept at 4 mg resulted in a faster decline in CMT in the first month after injection compared with conventional dose aflibercept at 2 mg. However, it did not achieve additional visual benefit. Therefore, the conventional dose of aflibercept can be selected for patients with refractory PCV and serous PED who have not been treated with fusion protein drugs.
In this study, no endophthalmitis, RPE tears, persistent ocular hypertension, or other complications were found during the follow-up period. However, short-term intraocular pressure increases occurred in two patients and four patients in the 2 mg aflibercept and 4 mg aflibercept groups, respectively. Intraocular pressure increases following intravitreal injection are mostly due to acute ocular hypertension occurring immediately after injection. However, this ocular hypertension is usually transient and well tolerated. It has been shown that ocular hypertension after intravitreal injection of anti-VEGF agents is associated with an increased total number of injections (more than 14 injections within 2 years and more than 20 injections within 3 years) [21].
It should be noted that replacement therapy is not limited to replacement therapy with monoclonal antibodies to fusion protein anti-VEGF drugs. The replacement treatment with ranibizumab may be equally effective [22-23] in patients with aflibercept-resistant AMD and PED.
The study was limited by the small sample size as well as by the short follow-up duration. Larger patient cohorts should be included, the follow-up time should be prolonged, and more comprehensive randomized controlled studies should be designed to verify the study results in the future. Whether the dose can be increased in patients with PCV and PED who are tolerant of conventional doses of aflibercept should also be further studied in a large-sample, prospective, and long-term follow-up study, so that better treatment strategies for patients with PCV and PED can be investigated.
Conflict of Interest
All authors declare that there is no conflict of interest
Contribution Statements of Authors
Zhou Pengyi: Experimental design, collection and analysis of data, article writing; Yang Lin, Xu Youmei, Pan Meng, Guo Ju: Collection and analysis of data; Du Liping: Experimental design, review and modification of article; Jin Xuemin: Experimental design, experimental guidance, review and modification of article
References
[1] Zhou N,Wei WB. Current advance of polypoidal choroidal vasculopathy in diagnosis and treatment [J].Chin J Exp Ophthalmol,2019,37 (1): 77-80. DOI: 10. 3760/cma. j. issn. 2095-0160. 2019. 01. 017
[2]Lee WK, lida T, Ogura Y, et al. Efficacy and safety of intravitreal aflibercept for polypoidal choroidal vasculopathy in the PLANET Study: a randomized clinical trial [J]. JAMA Ophthalmol, 2018,136 (7): 786-793. DOI: 10.lOOL/jamaophthalmol. 2018. 1804.
[3]Lim TH,Lai T, Takahashi K, et al. Comparison of ranibizumab with or without verteporfin photodynamic therapy for polypoidal choroidal vasculopathy: the EVEREST H randomized clinical trial [ J ]. JAMA Ophthalmol,2020,138(9): 935-942. DOI: 10. lOOL/jamaophthalmol. 2020.2443.
[4] Deng KY, Huang Z, Huang XL, et al. The e^ect of aflibercept in the treatment of exudative age-related macular degeneration combined with retinal pigment epithelial detachment [ J]. Chin J Ocul Fundus Dis, 2020,36(10): 764-771. DOI: 10. 3760/cma. j. cn511434-20200608- 00266.
[5] Treat-and-Extend Regimen for Management of Neovascular Age-related Macular Degeneration Chinese Experts Consensus Group. Chinese experts consensus of treat-and-extend regimen for management of neovascular age-related macular degeneration by intravitreal injection of aflibercept (2021) [ J ]. Chin J Exp Ophthalmol, 2021, 39 (7): 577-584. DOI:10.3760/cma.j. cnll5989-20210311-00162.
[6 ]Cheung C, Lai T, Teo K, et al. Polypoidal choroidal vasculopathy: consensus nomenclature and non-indocyanine green angiograph diagnostic criteria from the Asia-Pacific Ocular Imaging Society PCV Workgroup [J]. Ophthalmology, 2021,128 (3): 443 – 452. DOI: 10. 1016/j. ophtha. 2020.08.006.
[7] Kim K, Kim ES, Kim Y, et al. Outcome of intravitreal aflibercept for refractory pigment epithelial detachment with or without subretinal fluid and secondary to age-related macular degeneration [J]. Retina, 2019, 39(2): 303-313. DOI: 10. 1097/IAE. 0000000000001947.
[8 Kocak I. Intravitreal afLibercept in treatment-resistant pigment epithelial detachment [J].Int Ophthalmol, 2017,37 (3): 531 – 537. DOI: 10.100T/sl0792-016-0294-4.
[9] Yang L, Jin XM, Zhou PY. Intravitreal injection of bevacizumab or ranibizumab for the treatment of pathological myopia choroidal neovascularization [ J ]. Chin J Ocul Fundus Dis, 2017, 33 (2): 139-143. DOI:10. 3760/cma.j. issn. 1005-1015.2017.02.007.
[10] Khanani AM, Eichenbaum D, Schlottmann PG, et al. Optimal management of pigment epithelial detachments in eyes with neovascular age-related macular degeneration [J]. Retina, 2018, 38 (11): 2103-2117. DOI:10. 1097/IAE. 0000000000002195.
[11] Cheong KX,Grewal DS,Teo K,et al. The relationship between pigment epithelial detachment and visual outcome in neovascular age-related macular degeneration and polypoidal choroidal vasculopathy [ J ]. Eye (Lond), 2020, 34 (12): 2257 – 2263. DOI: 10. 1038/s41433-020- 0803-6.
[12] Au A, Parikh VS, Singh RP, et al. Comparison of anti-VEGF therapies on fibrovascular pigment epithelial detachments in age-related macular degeneration [J]. Br J Ophthalmol,2017; 101 (7): 970-975. DOI:10.1136/bjophthalmol-2016-309434.
[13] Broadhead GK, Hong T, Zhu M, et al. Response of pigment epithelial detachments to intravitreal afLibercept among patients with treatmentresistant neovascular age-related macular degeneration [J]. Retina, 2015,35(5): 975-981. DOI: 10.1097/IAE. 0000000000000409.
[14] You QS, Gaber R, Meshi A, et al. High-dose high-frequency afLibercept for recalcitrant neovascular age-related macular degeneration [J]. Retina,2018,38(6): 1156-1165. DOI: 10.1097/IAE. 0000000000001726.
[15] Inoue M, Arakawa A, Yamane S, etaLVariable response of vascularized pigment epithelial detachments to ranibizumab based on lesion subtypes, including polypoidal choroidal vasculopathy [ J ]. Retina, 2013,33(5): 990-997. DOI: 10.1097/IAE. 0b013e3182755793.
[16] Romanek J, Palyzova H, Grygar J, et al. AfLibercept for vascularised serous pigment epithelial detachment: one-year anatomical and functional results[ J]. Cesk Slov Oftalmol, 2020,76 (2): 88-93. DOI: 10.31348/2020/16.
[17] Chan CK, Sarraf D, Abraham P. Treatment outcomes of conventional or high-dose ranibizumab for vascularized pigment epithelial detachment based on lesion subtypes [J]. Eur J Ophthalmol, 2018, 28 (6): 677-683. DOI: 10. 1177/1120672117747034.
[18]Jaffe GJ, Martin DF, Toth CA, et al. Macular morphology and visual acuity in the comparison of age-related macular degeneration treatments trials [J]. Ophthalmology, 2013 ? 120 (9): 1860-1870. DOI: 10.1016/ j.ophtha.2013.01.073.
[19]Jaffe GJ, Ying GS,Toth CA,et al. Macular morphology and visual acuity in year five of the comparison of age-related macular degeneration treatments trials [J]. Ophthalmology, 2019,126 (2): 252 – 260. DOI: 10.1016/j. ophtha. 2018.08.035.
[20]Cheong KX, Teo K, Cheung C. Influence of pigment epithelial detachment on visual acuity in neovascular age-related macular degeneration [ J ]. Surv Ophthalmol, 2021,66 (1): 68 – 97. DOI: 10. 1016/j. survophthal. 2020.05.003.
[21]Levin AM,Chaya CJ, Kahook MY, et al. Intraocular pressure elevation following intravitreal anti-VEGF injections: short- and long-term considerations [ J ].J Glaucoma, 2021,30 (12): 1019- 1026. DOI: 10.1097/IJG. 0000000000001894.
[22]Davoudi S, Roohipourmoallai R, Guerin CM, et al. Exacerbation of pigment epithelial detachment following aflibercept: a case of bevacizumab rescue [ J/OL ]. Am J Ophthalmol Case Rep, 2021,24: 101216[ 2022 – 06 – 10 ]. http: //www. ncbi. nlm. nih. gov/puhmed/ 34693076. DOI: 10. 1016/j. ajoc. 2021.101216.
[23] Marquis LM, Mantel I. Beneficial switch from aflibercept to ranibizumab for the treatment of refractory neovascular age-related macular degeneration [J].Graefe V Arch Clin Exp Ophthalmol, 2020,258 (8): 1591-1596. DOI: 10.1007/s00417-020-04730-8.