·Case Report·
Uveitis complicated with systemic tuberculosis associated with inappropriate adalimumab therapy: two case reports
Sun Ludan, Zhu Jie, Zhu Jun, Yu Jing, Chen Fang
Department Of Ophthalmology, Northern Jiangsu People’s Hospital of Jiangsu Province, Yangzhou 225001, China
Corresponding author: Chen Fang, Email: cfyzsbyy@163.com
Fund program: Yangzhou Science and Technology Development Plan Project (YZ2020112)
DOI: 10.3760/cma.j.cn115989-20220506-00196
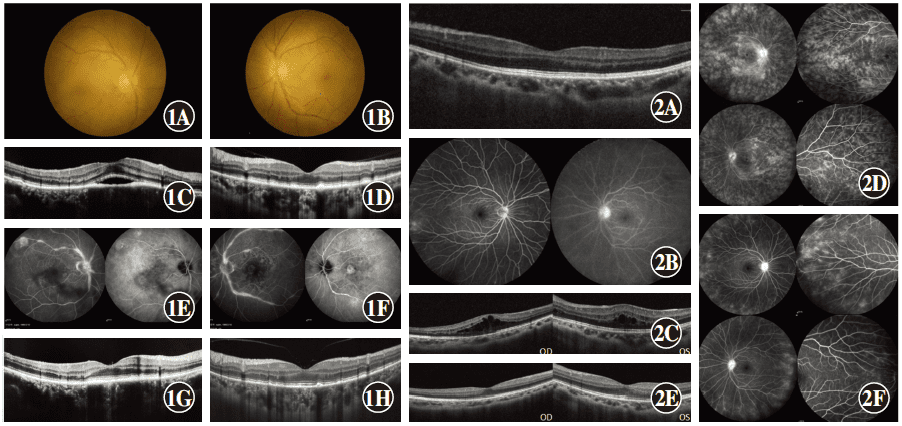
Case 1: A 56-year-old male was admitted to the Respiratory Department of Subei People’s Hospital affiliated to Yangzhou University on May 28, 2020 due to cough and fever. The patient was diagnosed with bilateral Harada disease at another hospital 3 months prior, and was successively treated with systemic glucocorticoid, cyclosporine, and adalimumab, but still had recurrent attacks. The Respiratory Department requested an ophthalmic consultation. The best-corrected visual acuity (BCVA) was 0.3 in the right eye and 1.0 in the left eye. The corneas of both eyes were clear, while keratic precipitates (KP) and mild vitreous opacity were present in both eyes. Retinal edema was present in the posterior pole in the right eye (Fig. 1A), and fundus of the left eye revealed slightly tortuous dilatation of the retinal vein in the posterior pole (Fig. 1B). Optical coherence tomography (OCT) indicated hyperreflective deposition in the retinal pigment epithelium (RPE) layer in both eyes and serous retinal detachment in the macular region of the right eye (Fig. 1C, D). Fundus fluorescein angiography (FFA) and indocyanine green angiography (ICGA) revealed multifocal choroiditis and retinal vasculitis in both eyes (Fig. 1E, F). Examinations after admission showed that the t cell spot test (T-SPOT) was positive, and a bronchoscopic biopsy indicated tuberculosis of the left main lobes, left upper, and lower lobes (inflammatory infiltration, which was a type of granulation hyperplasia). Mycobacterium tuberculosis DNA in the patient’s sputum was positive. Consultation with the Yangzhou Infectious Diseases Hospital Tuberculosis Department indicated that the patient had active tuberculosis, and we concluded that his diagnosis was active pulmonary tuberculosis with bilateral tuberculosis-associated uveitis. After a detailed analysis of his medical history, we found that his T-SPOT result was positive before the use of glucocorticoid treatment, when his preliminary diagnosis was Harada disease, which had not been noted. The patient was then transferred to the infectious disease hospital for regular anti-tuberculosis treatment with withdrawal of glucocorticoid, cyclosporine, and adalimumab. Two weeks after tuberculosis treatment, his bilateral BCVAs were both 1.0, there was no anterior chamber flare in his right eye, and OCT showed that serous retinal detachment in the macular region of the right eye recovered (Fig. 1G). Three months after regular anti-tuberculosis treatment, the lesions of pulmonary active tuberculosis were stable, his BCVAs were maintained at 1.0 in both eyes, and the high reflex substance of the RPE layer in both eyes decreased, when compared to that before treatment. After nearly 1 year of regular anti-tuberculosis treatment,
the patient stopped taking all medicines and there was no recurrence during a 1-year follow-up.
Case 2: A 28-year-old female was admitted on March 16, 2021 for relapsing redness in both eyes and blurred vision for 1 month. One year prior, the patient was referred to our hospital because of iridocyclitis in the left eye. At that time, FFA showed high fluorescence only in the optic disc of the left eye, and OCT revealed no unusual characteristics (Fig. 2A, B). The patient’s serum HLA-B27 was positive, and sacroiliac arthritis was indicted using sacroiliac joint computerized tomography (CT). The patient was diagnosed with ankylosing spondylitis (AS) by the Rheumatology Department, and was recommended for treatment using biological agents. Before treatment, routine tests showed a positive T-SPOT in the serum, the purified protein derivative was moderately positive, and there was no abnormality using CT of the chest. Two months after prophylactic anti-tuberculosis treatment, the patient was treated with adalimumab by a rheumatologist, and treatment with anti-tuberculosis drugs was terminated. Six months after adalimumab was prescribed, the patients began to have the symptoms of relapsing redness, blurred vision in both eyes, with no alleviation using tobramycin dexamethasone eye drops.
After admission, examinations showed that her BCVAs were 0.5 in both eyes, with dust-like KP, aqueous flare, mild vitreous opacity, and mild optic disc hyperemia present in both eyes, while there was no lesion in the retina. OCT revealed retinal thickening and cystoid macular edema in the posterior pole of both eyes (Fig. 2C). FFA showed diffuse fluorescein leakage of retinal vessels in both eyes, and late phase cystoid macular edema, suggesting bilateral retinal vasculitis (Fig. 2D). Her serum T-SPOT was still positive, while there was no abnormality using CT of the chest. She was then diagnosed with bilateral tuberculosis-associated uveitis. After adalimumab withdrawal, she was transferred to the infectious disease hospital for regular anti-tuberculosis treatment without the use of glucocorticoid and other immunosuppressants. At the 2-week follow-up, her clinical symptoms improved, her BCVAs were 1.0 in both eyes, and the vitreous opacity was significantly decreased. OCT indicated that neuroepithelial edemas in the macular areas of both eyes were significantly recovered (Fig. 2E). Three months following regular anti-tuberculosis treatment, FFA showed a significant improvement of retinal vasculitis in both eyes (Fig. 2F), and OCT revealed that the macular edema had disappeared in both eyes. At present, there has been no recurrence of bilateral uveitis in the course of treatment with isoniazid and rifampicin.
Case 1 Examination of images A: Fundus of the right eye showed mild retinal edema in the posterior pole. B: Fundus of the left eye. C: There was hyperreflective deposition in the retinal pigment epithelium (RPE) layer with serous retinal detachment in the central fovea of the macula in the right eye. D: There was hyperreflective deposition in the RPE layer of the left eye. E, F: Bilateral fundus fluorescein angiography/indocyanine green angiography revealed multifocal choroiditis, retinal vasculitis (E: right eye; F: left eye). G: Serous detachment of the macular region in the right eye recovered 2 weeks after anti-tuberculosis treatment. H: High reflectance of the RPE layer of the left eye decreased 3 months after anti-tuberculosis treatment.
Case 2 Examination of images A: There was no abnormal optical coherence tomography reflex in the left eye at initial visit. B: Left optic disc hyperfluorescence was found at initial visit. C: Bilateral cystoid macular edema. D: Bilateral retinal vascular diffuse fluorescein leakage, cystoid macular edema in the late phrase. E: Bilateral neuroepithelial edema improved 2 weeks after anti-tuberculosis treatment, and there was no macular edema. F: Bilateral vascular fluorescein leakage decreased 2 months after anti-tuberculosis treatment.
Discussion: Adalimumab is the only tumor necrosis factor-α (TNF-α) inhibitor approved by the Food and Drug Administration (FDA) (U.S.) for non-infectious uveitis. With the wide use of TNF-α inhibitors in the treatment of refractory uveitis, the quality of life and prognoses of patients with non-infective uveitis have significantly improved[1]. TNF-α plays a central role in the control of tuberculosis infection, while it also has the highest risk of activating tuberculosis infection among numerous biological agents[2]. China has a high prevalence of tuberculosis, so Chinese clinicians need to focus on the management of TNF inhibitors for the treatment of tuberculosis [3-4]. Chinese rheumatologists issued the “Expert Consensus on the Prevention and Management in the Application of TNF antagonists in Tuberculosis” in 2013[5], to guide clinicians in the screening and management of tuberculosis during use of TNF antagonists.
Studies have shown that patients with latent tuberculosis infection treated with TNF-α inhibitors have approximately a four-fold increase in the risk of developing active tuberculosis, when compared with healthy controls. For patients with latent tuberculosis and obsolete pulmonary tuberculosis, expert consensus recommends that prophylactic anti-tuberculosis treatment is essential before the use of TNF-α antagonists. TNF-α antagonists should be used at least 4 weeks after receiving prophylactic anti-tuberculosis therapy. At present, there is no worldwide unified plan for the choice of drugs and the course of treatments for prophylactic anti-tuberculosis treatment. In China, prophylactic monotherapy is not suggested, while continuous combined therapy for at least 6 months is recommended. In our study, both patients were found to have positive serum T-SPOT before use of TNF-α inhibitors, but they did not receive standard anti-tuberculosis treatment. Adverse reactions occurred after adalimumab administration, and Case 1 even developed active pulmonary tuberculosis. Case 1 was initially diagnosed as Harada disease. According to fundus images of the initial hospital provided by the patient, FFA showed pinpoint strong fluorescence in the early phases in the posterior pole of both eyes, and subretinal fluorescence accumulated in the late phase. OCT showed multiple neuroepithelial detachments and wave-like changes of the RPE layer in both eyes, which was consistent with symptoms of Harada disease. However, the primary physician ignored the positive results of T-SPOT at the first visit and the patient did not receive prophylactic anti-tuberculosis treatment. The patient responded poorly to high dose steroid pulse therapy, then was treated with combined cyclosporine and adalimumab. After 2 months, the patient showed active pulmonary tuberculosis, and the fundus showed bilateral multifocal choroiditis with retinal vasculitis. We thought that it was tuberculosis-associated uveitis, then we found that the patient’s condition included the lung and eyes, which improved after simple regular anti-tuberculosis treatment. Although the use of glucocorticoids and cyclosporine may lead to aggravation or resurgence of tuberculosis, patients may avoid serious adverse reactions such as active tuberculosis if routinely screened for tuberculosis and given anti-tuberculosis treatment before the use of adalimumab. Case 2 was initially diagnosed as AS with anterior uveitis, and latent tuberculosis infection was found by screening before adalimumab treatment. The patient was treated with adalimumab after 2 months of anti-tuberculosis treatment. At the same time during anti-tuberculosis treatment withdrawal, recurrent uveitis occurred in both eyes after 6 months, and the fundus examination revealed bilateral retinal vasculitis with macular edema, which could not be explained by AS with uveitis. The serum T-SPOT result was positive when we retested, so we considered it as tuberculous panuveitis, and the patient’s fundus lesions also improved after simple regular anti-tuberculosis treatment. Overall, the present study suggested careful consideration of indications for the use of biological agents such as adalimumab in the treatment of uveitis, followed by standardized screening before treatment.
Active tuberculosis can occur at any time after TNF-α inhibitor therapy is initiated. Case 1 and Case 2 developed tuberculosis-associated uveitis 2 months and 6 months, respectively, after adalimumab treatment, which suggested that all patients with latent tuberculosis or with a history of tuberculosis treatment should be closely followed-up after initiating TNF-α inhibitor therapy. It is therefore recommended that clinicians monitor patients’ symptoms and signs, chest X-rays, and a serum T-SPOT of active tuberculosis at 3 and 6 months after the start of TNF-α inhibitor therapy, and every 6 months thereafter until 3 months after drug withdrawal. The patients’ tuberculosis infections were activated after treatment with biological agents, whose main site of infection was extrapulmonary tuberculosis. Hernanz et al.[7] reported that a patient with AS combined with uveitis developed extrapulmonary active tuberculosis after adalimumab treatment, and a good prognosis was obtained after terminating administration of adalimumab and initiating anti-tuberculosis therapy. In the present study, the two patients also showed good prognoses after stopping alimumab and receiving regular anti-tuberculosis therapy. Therefore, if recurrent uveitis that cannot be explained by primary disease is found in patients treated with adalimumab, tuberculosis-related indicators should be carefully monitored to eliminate its association with tuberculosis Timely detection of tuberculosis-associated uveitis and treatment can therefore avoid serious damage to the visual function of patients.
In conclusion, ophthalmologists should recognize and understand adverse reactions related to TNF-α inhibitors and their pathogenesis, and should therefore prescribe TNF-α inhibitors in a judicious manner. For patients with non-infective uveitis treated with TNF-α inhibitors, it is necessary to standardize disease screening and prevention and control measures, before and after medication use, as well as monitor drug use to minimize or even avoid the occurrence of adverse drug reactions.
References
[1] LaMattina KC, Goldstein DA. Adalimumab for the treatment of uveitis[J]. Expert Rev Clin Immunol, 2017, 13(3):181-188. DOI: 10.1080/1744666X.2017.1288097.
[2] Gómez-Reino JJ, Carmona L, Valverde VR, et al; BIOBADASER Group. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant
increase in tuberculosis risk: a multicenter active-surveillance report[J]. Arthritis Rheum, 2003, 48(8):2122-2127. DOI: 10.1002/art.11137.
[3] Cantini F, Nannini C, Niccoli L, et al. Guidance for the management of patients with latent tuberculosis infection requiring biologic therapy in rheumatology and dermatology clinical practice[J]. Autoimmun Rev, 2015, 14(6): 503-509. DOI: 10.1016/j.autrev.2015.01.011.
[4] Chi Y, Yang L. Pay attention to the risk of infection and tumor progression during application of TNF-α inhibitors in uveitis[J]. Chin J Exp Ophthalmol, 2021, 39(11):929-932. DOI: 10.3760/cma.j.cn115989-20211020-00571.
[5] TNF-antagonist use in tuberculosis prevention and management expert recommended group. Expert consensus on prevention and management of tuberculosis in the application of tumor necrosis factor antagonist [J]. Chin J Rheumatology, 2013, 17(8):508-512. DOI: 10.3760/cma.j.issn.1007-7480.2013.08.002.
[6] Baddley JW, Cantini F, Goletti D, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Soluble immune effector molecules [I]: anti-tumor necrosis factor-α agents)[J]. Clin Microbiol Infect, 2018, 24 Suppl 2:S10-S20. DOI: 10.1016/j.cmi.2017.12.025.
[7] Hernanz I, Miguel Escuder L, Chamorro L, et al. Tuberculosis-related uveitis in patients under anti-TNF-alpha therapy: a case series[J/OL]. Ocul Immunol Inflamm, 2022, 30(4):839-844. https://pubmed.ncbi.nlm.nih.gov/33216652/. DOI: 10.1080/09273948.2020.1834588.