·Clinical Research·
Association between visual function and optic fiber layer thickness after gene therapy for Leber hereditary optic neuropathy
Yuan Jiajia1, Zhang Yong2, Chen Changzheng1, Yang Xueying1, Miao Qingmei1, Kai Yoon Fan3, Li Bin2,3
1Department of Ophthalmology, People’s Hospital of Wuhan University, Wuhan 430065, China; 2Department of Ophthalmology, Taihe Hospital, Hubei University of Medicine, Hubei, 442000, China; 3Wuhan Neurophth Biological Technology Co., Ltd., Wuhan 420200, China
Corresponding author: Li Bin, Email: libin-12@163.com
[Abstract] [View PDF in English] [View PDF in Chinese] [Read Full Text]
Objective To investigate the association between the rehabilitation of visual function and retinal nerve fiber layer (RNFL) thickness in Leber hereditary optic neuropathy (LHON) patients receiving gene therapy for the disease.
Methods A multi-center, non-randomized, single-arm clinical trial was conducted. A total of 159 LHON patients were enrolled at Tongji Hospital, Tongji Medical College, Huazhong University of Science & Technology, Shiyan Taihe Hospital and Ezhou Central Hospital from December 2017 to December 2018. All patients were administered with a single unilateral intravitreal injection (0.05 μL) of recombinant adeno-associated virus 2 carrying reduced nicotinamide adenine dinucleotide dehydrogenase subunit 4 (rAAV2-ND4) and were followed up before and 1, 3, 6, and 12 months after treatment. The best-corrected visual acuity (BCVA) converted to the logarithm of minimal angle of resolution (logMAR) was assessed with a standard logarithmic visual acuity chart. Perimetry indicators including visual field index (VFI) and mean deviation (MD) were measured with a Humphrey Field Analyzer. RNFL thicknesses in the superior, inferior, temporal, and nasal optic disc, and the average RNFL thickness were detected with a Spectralis® HRA+OCT. The 12-month postoperative BCVA, visual field, and RNFL thickness were taken as the primary outcomes. According to the improvement of BCVA, VFI, and MD at 12 months after therapy, there were 81 vision improved eyes after injection, 62 vision unimproved eyes after injection, 65 vision improved eyes without injection, 78 vision unimproved eyes without injection, 48 VFI improved eyes with injection, 71 VFI unimproved eyes with injection, 47 VFI improved eyes without injection, 72 VFI unimproved eyes without injection, 52 MD improved eyes with injection, 67 MD unimproved eyes with injection, 47 MD improved eyes without injection, and 72 MD unimproved eyes without injection. The correlations between BCVA, VFI, and MD with RNFL thicknesses were evaluated using Pearson’s linear correlation analysis. This study adhered to the Declaration of Helsinki. The study protocol was approved by the Ethics Committees of Wuhan Tongji Hospital (No. TJ-IRB20180316), Taihe Hospital of Shiyan (No. 2017-01), Ezhou Central Hospital (No. 2017-K-05), and the People’s Hospital of Wuhan University (No. WDRY2020-K202). Written informed consent was obtained from each patient or custodian prior to entering the study cohort. Results Among the patients receiving rAAV-ND4 gene therapy, the 12-month postoperative BCVA was 1.37±0.55, which was significantly better than 1.70±0.41 before treatment (baseline) of the injected eyes (t=4.920, P<0.001). Significant improvements between 12-month postoperative BCVA and baseline BCVA were also found in the uninjected eyes of the patients (t=3.550, P<0.001). The 12-month postoperative VFI of the patients was significantly improved and the 12-month postoperative MD of the patients was significantly decreased in comparison with those at baseline in the injected eyes (both at P<0.001). Similar improvements of VFI and MD were observed in the uninjected eyes (both at P<0.01). The RNFL of patients was thinner after therapy. In the vision improved eyes with injection, the BCVA was negatively correlated with superior, inferior, temporal, nasal, and average RNFLs (r=−0.362, −0.292, −0.307, and −0.308, respectively; all at P<0.05). In the VFI improved eyes with injection, VFIs were positively correlated with superior, inferior, nasal, and average RNFLs (r=0.439, 0.356, 0.294, and 0.401, respectively; all at P<0.05). In the MD improved eyes with injection, the MDs were correlated in the superior, inferior, nasal, and average RNFLs (r=0.495, 0.424, 0.377, and 0.474, respectively; all at P<0.05).
Conclusions The recovery of visual function was associated with RNFL thickness after the intravitreal injection of rAAV2-ND4 in LHON eyes. Recovery of visual acuity was better in eyes with thicker RNFLs.
[Key words] Gene therapy; Optic atrophy, hereditary, Leber/therapy; Visual acuity; Visual fields; Retinal nerve fiber layer thickness; Human; Tomography, Optical coherence; Recombinant adeno-associated virus; NADH dehydrogenase subunit 4
Fund program: National Natural Science Foundation of China (82101115, 81770969); Wuhan University Independent Innovation Fund Youth Project (2042021kf0094)
Trial registration: Clinical Trials.gov, NCT03153293
DOI: 10.3760/cma.j.cn115989-20210409-00242
Leber hereditary optic neuropathy (LHON) is a rare matrilineal hereditary disease caused by point mutations in mitochondrial DNA1. The main clinical manifestations include an acute or subacute, and simultaneous or sequential decrease in binocular visual acuity to less than 0.1, decreased light sensitivity, and visual field and central visual field defects. The common LHON gene mutation site is 11778G>A in the nicotinamide adenine dinucleotide plus hydrogen dehydrogenase subunit 4 (ND4) gene, which is induced by retinal ganglion cells (RGCs). When the structure and function of the respiratory chain in the energy metabolism of RGCs are impaired, adenosine triphosphate generation is blocked, and cell metabolism suffers from energy deficiency and oxidative stress, thus leading to the apoptosis of RGCs and atrophy and thinning of the retinal nerve fiber layer (RNFL); the visual function of the affected eye is also significantly impaired2-4. Visual impairment and visual field defects are the main visual function changes in LHON patients, and changes in RNFL are significant in the retinal nerve tissue structure of LHON patients5-6. According to international clinical staging standards, LHON can be divided into four stages: Stage 0 is considered asymptomatic (mutation carrier). Stage 1 is subacute and generally occurs 6 months after disease onset. Stage 2 is a dynamic change period that mainly occurs 6‒12 months after disease onset. Stage 3 is chronic and mainly occurs 12 months after disease onset7. Our previous study on RNFL thickness characteristics during different stages of LHON reported that RNFL edema gradually worsened in the acute stage, optic nerve atrophy occurred in the chronic and plateau stages, and RNFL thinning gradually stabilized 6. However, few studies have reported correlations between the recovery of visual function before and after gene therapy and the RNFL thickness, which is important for assessing clinical efficacy after treatment and for understanding factors affecting the recovery of the visual field.
There is currently no effective treatment for LHON. In recent years, gene therapy for LHON has developed rapidly because relevant clinical research has been performed worldwide8-10. We started a clinical study of LHON gene therapy on December 27, 2017, and first reported relevant clinical research results at home and abroad11-13. However, relevant clinical studies in China are still in the initial stages, and major research results on the effects of gene therapy, particularly the correlation between visual function recovery and retinal structure in patients after gene therapy, is lacking. The current study therefore further identified correlations between visual acuity and visual field recovery after LHON gene therapy, and showed that the RNFL thickness can serve as a reference for the assessment of clinical efficacy of gene therapy for LHON, and for the optimization of treatment plans.
1 Results and methods
1.1 General Information
This study used the open label, single arm, multicenter clinical trial method developed by Tongji Hospital affiliated with Tongji Medical School, Huazhong University of Science and Technology; Taihe Hospital, Shiyan City, Hubei; Ezhou Central Hospital affiliated with Wuhan University; People’s Hospital of Wuhan University; and Mr. Eye Hospital, San Pedro, Buenos Aires, Argentina. A total of 159 patients with LHON were recruited from December 2017 to December 2018. The inclusion criteria were as follows: (1) patients with LHON diagnosed with the m11778G-A/MT-ND4 mutation via genetic testing, (2) ages of 10-60 years, (3) best-corrected visual acuity (BCVA)<0.3, and (4) a general condition that could tolerate a series of eye examinations and treatments, and which could be followed-up with time. The exclusion criteria were as follows: (1) presence of other ocular diseases that may affect the function of the optic nerve, such as glaucoma, optic nerve disease, or systemic disease; (2) use of drugs that may affect the accuracy of the study, such as aidibenquinone, vitamins, antioxidants, or traditional Chinese medicine, within 6 months prior to gene therapy; and (3) patients with spontaneous visual acuity improvement during the natural course of observation 1 year before treatment. The 159 patients with LHON included 143 males and 16 females, including 10 white males from Argentina. All patients had binocular disease. The patients’ ages ranged from 6-45 years, with an average age of 19.2±7.2 years. The course of the disease ranged from 3-312 months. This study was conducted by Tongji Hospital Affiliated with Tongji Medical College, Huazhong University of Science and Technology (approval no. TJ-IRB20180316); Taihe Hospital, Shiyan City, Hubei (approval no. 2017-01); Ezhou Central Hospital (approval no. 2017-K-05); and the People’s Hospital of Wuhan University (approval no. WDRY2020-K202). This study was approved by the appropriate ethics committee in accordance with the Declaration of Helsinki and was registered in ClinicalTrials.gov prior to study implementation. All patients or authorized guardians were fully aware of the medical treatment process, protocol of the study, and purpose of the study, and voluntarily signed the informed consent forms.
1.2 Methods
1.2.1 Intravitreal injection Recombinant adenoassociated virus 2 carrying ND4 (rAAV2-ND4) was used for intravitreal injection (Wuhan Newfors Biotechnology, Wuhan, China), and 0.05 mL monocular vitreous injection was performed. The eye with poorer BCVA was selected for injection. If both eyes had the same vision, the right eye was selected as the injection eye. Vitreous cavity injection was performed in the operating room. The patient was placed in a supine position, and propimexaine hydrochloride eye drops were applied three times to the eye. Routine disinfection was performed for the face and eye by using a towel. The eyelid was opened with an eyelid opener, the conjunctival sac was rinsed with 0.5% povidone-iodine disinfectant, and the conjunctival sac was rinsed with normal saline after 10 s. A 1 mL syringe was used to draw 0.05 mL rAAV2-ND4 (1.0 × 1010 vg). The needle was injected 4 mm behind the corneal limbal, clockwise from the 7 o’clock to 8 o’clock positions, and the drug was slowly injected into the eye. The needle was then removed, and a sterile cotton swab was pressed at the injection site for 2 min. Antibiotic ointment was then applied to the operated eye, which was bandaged. The patient was then placed in a supine position for 30 min and returned to the ward. All surgeries were performed by the same physician.
1.2.2 Ophthalmic examination All enrolled patients were followed up for at least 1 year before gene therapy, and the BCVA, visual field, and RNFL thickness were examined by the same physician before treatment and at 1, 3, 6, and 12 months after treatment without knowledge of the injection and non-injection eyes.
1.2.2.1 BCVA examination The standard logarithmic visual acuity chart (Wenzhou Xingkang Medical Technology, Wenzhou, China) was used to examine the patients’ visual acuities. The distance between the inspected eye and the visual acuity chart was 2.5 m. The binocular visual acuity was measured, and the BCVA was converted to logarithm of minimal angle of resolution (logMAR) visual acuity for statistical analysis. BCVA at the last preoperative follow-up was used as the baseline visual acuity.
1.2.2.2 Visual field examination The binocular visual field of the patient was measured using a Humphrey automatic visual field meter (740i; Carl Zeiss, Jena Germany). The device was set to a 30-2 central threshold test in the SITA rapid detection mode.
1.2.2.3 RNFL thickness measurement RNFL thickness measurements were performed using the Spectralis® HRA+OCT Glaucoma-ONH mode (Heidelberg Engineering, Heidelberg, Germany) to determine the superior, inferior, temporal, nasal, and average RNFL thicknesses from the inner boundary membrane layer to the photoreceptor cell layer.
1.2.3 End point evaluation indexes The main evaluation indexes were postoperative visual acuity, visual field, and RNFL thickness changes. (1) Changes in visual acuity: improvement in BCVA at 12 months after injection was regarded the outcome of visual acuity after treatment. Improved visual acuity was defined as an increase in BCVA ≥ 0.3, and no improvement in visual acuity was defined as an increase in BCVA<0.3. According to BCVA improvement at 12 months after injection, the patients were divided into 81 eyes with improved visual acuity after injection, 62 eyes with no improvement in visual acuity after injection, 65 eyes of uninjected patients with no improvement in visual acuity after injection, and 78 eyes of uninjected patients with no improved visual acuity after injection. (2) For changes in the visual field, visual field improvement at 12 months after injection was used as the outcome of the visual field, with the visual field index (VFI) increasing by 10% and the mean deviation (MD) increasing by 3 dB as the improvement standard. Patients were divided into 48 eyes with improved VFI injection, 71 eyes with no improved VFI injection, 47 eyes with no improved VFI injection, and 72 eyes with no improved VFI injection. There were 52 eyes with improved MD injections, 67 eyes with no improved MD injections, 47 eyes with no improved MD injections, and 72 eyes with no improved MD injections. (3) The dynamic changes in RNFL were followed up at 1, 3, 6, and 12 months after injection to determine changes in RNFLs in the injected and non-injected eyes at different time points after injection. (4) The relationship between visual function indicators and the RNFL 12 months after treatment was evaluated.
1.3 Statistical methods
SPSS statistical software for Windows, version 24.0 (SPSS, Chicago, IL, USA) was used for statistical analysis. Shapiro-Wilk tests were used to determine if the measurement data were normally distributed, together with ±s, Levene’s tests, and linear f-tests. The differences among BCVA, VFI, and MD before and after treatments were compared using a paired t-test. The RNFL thicknesses of different parts at different time points before and after treatments were compared using a generalized linear mixing model. Pearson’s correlation coefficient was used to evaluate correlations between BCVA, VFI, MD, and RNFL thicknesses at end points after treatment. P<0.05 was considered statistically significant.
2 Results
2.1 Comparison of BCVA in patients with improved vision before and after treatment
A total of 91 patients (57.23%) had improved BCVA 12 months after treatment (both in the injected and non-injected eyes). After treatment, BCVA increased by 0.31 ± 0.40 compared with that before treatment, and the difference was statistically significant (t=4.920, P<0.001). BCVA in eyes without injection increased by 0.23±0.46 on average compared with that before and after treatment, and the difference was statistically significant (t=3.550, P<0.001; Table 1).

2.2 Comparison of VFI before and after treatment
At 12 months after treatment, VFI was significantly increased in both injected and non-injected eyes (t=−4.451, P<0.001), and the MD was significantly decreased, when compared with that before treatment (t=−5.110, P<0.001). After 12 months of treatment, the VFI of patients without injection was significantly higher than that before treatment (t=−3.352, P=0.001), and the MD was significantly decreased (t=−2.745, P=0.007; Table 2).

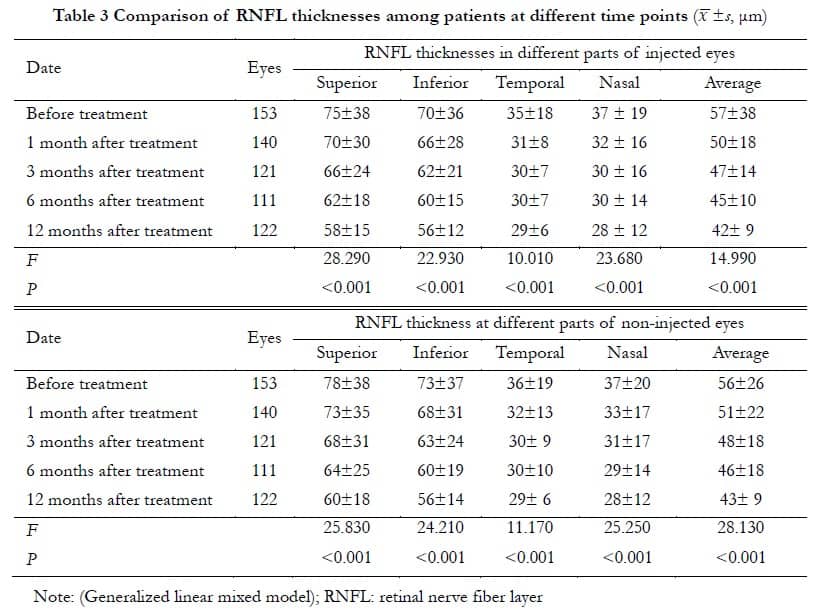
2.3 Comparison of RNFL thicknesses before and after treatments
The RNFL thicknesses at 1, 3, 6, and 12 months before and after treatments are shown in Table 3, which showed that the overall RNFL thickness gradually decreased after treatment. There was a significant difference in RNFL thicknesses between injected and non-injected eyes before and after treatment (all at P<0.001).
2.4 Correlation analysis between BCVA and RNFL thicknesses in patients after treatment
BCVA was negatively correlated with thicknesses of the superior, inferior, nasal, and average RNFLs of the retina (r=−0.362, −0.292, −0.307, and −0.308, respectively; all at P<0.05). BCVA was positively correlated with the thickness of the superior RNFL (r=0.066, P=0.003) and negatively correlated with the thickness of the nasal side and average RNFL thicknesses (r=−0.264 and −0.279, respectively; both at P<0.05). There was no significant correlation between BCVA and RNFL thicknesses in the non-injected eye improvement group (all at P>0.05; Table 4).

2.5 Correlation analysis of VFI, MD, and RNFL thicknesses after treatment
There was a positive correlation between the VFI of the injected eye and VFI of the non-injected eye, and there was a positive correlation between VFI and the average RNFL thicknesses of the above, below, nasal side, and other groups (r=0.439, 0.356, 0.294, and 0.401, respectively; all at P<0.05); in the group without increased VFI (r=0.301, 0.276, 0.339, and 0.366, respectively; all at P<0.05); and in the non-injection VFI increased group (r=0.580, 0.524, 0.570, and 0.643, respectively; all at P<0.05). There was no significant correlation between VFI and RNFL thickness in the group with no increase in VFI (all at P>0.05; Table 5).There was a positive correlation between the MD thickness above and below the nasal side and the average RNFL thicknesses in the increased ocular MD injection group, no increased ocular MD injection group, and no increased ocular MD injection group (r=0.495, 0.424, 0.377, and 0.474, respectively; all at P<0.05); in the injection eye MD group (r=0.314, 0.292, 0.333, and 0.377, respectively; all at P<0.05); and in the group without ocular MD injection (r=0.601, 0.601, 0.573, and 0.665, respectively; all at P<0.05). There was a positive correlation between the MD and average RNFL thickness in the non-injection group (r=0.280, P<0.05) (Table 6).

3 Discussion
We performed the first clinical trial of LHON gene therapy from 2011 to 2012 and analyzed the improvement in the visual field of nine patients before and after surgery. Nine patients completed the clinical follow-up 90 months after surgery, and six patients continued to improve their visual acuity, thus showing the long-term effectiveness of gene therapy 14-17. The team conducted a second clinical study of gene therapy in December 2017, in which 159 patients with LHON were treated, but the vision of some patients did not improve. In addition to age, disease course, and baseline BCVA before treatment, improvements in vision and visual field were correlated with RNFL thickness after gene therapy 11,13. Together, the results of this study showed that BCVA, VFI, and MD were positively correlated with RNFL thicknesses in the injection eye vision improvement group following gene therapy. A thicker RNFL after gene therapy corresponded to a better prognosis of vision and the visual field; this correlation reflected the effect of LHON gene therapy and delayed atrophy of the optic nerve and preserved or improved existing vision. Clinical follow-up after gene therapy was therefore important for identifying factors associated with gene therapy.
The central vision represents the optic nerve and retinal function of the macular papillary tract, and the visual field directly reflects the optic nerve and retinal function of the macular papillary tract. The structure of the optic nerve determines the BCVA and visual field of patients with LHON, and mitochondrial dysfunction caused by gene mutations can lead to changes in optic nerve thickness 6. Patients with LHON first experience acute BCVA decline and loss of central visual field, which then gradually expands outward. The loss of the visual field follows the rule of optic nerve loss 18. The temporal and inferior quadrant RNFL thickens in the asymptomatic and acute phases and gradually thins in the temporal quadrant as the disease progresses. The RNFL in the superior, nasal, and inferior quadrants of the optic disc in the chronic stage also gradually becomes thinner and generally reaches stability 1 year after onset.
The principle of gene therapy for LHON is that recombinant adeno-associated virus 2 containing the normal ND4 gene, transcribes and translates normal ND4 protein in mitochondria and RGCs, to continuously supplement the energy needed by RGCs and prevent further atrophy because of lack of energy. Visual field recovery after gene therapy was therefore negatively correlated with optic nerve injury, and the optic nerve with less damage recovered first after treatment.
Overall, the present study showed that visual acuity improvement of the injected eye was negatively correlated with the superior, inferior, nasal, and average RNFL thicknesses, but was not significantly correlated with the temporal RNFL thickness. This is inconsistent with our understanding that the thickness of the macular papillary tract RNFL determines visual acuity, and is worthy of further investigation. Given that the principle of monocular injection is to treat eyes with poor vision, the right eye should be treated if both eyes have the same vision. The RNFL thickness in the injected eye before treatment was thinner than that in the non-injected eye. The results of this study therefore explained the protective effect of gene therapy on the optic nerve. However, because of a lack of a detailed detection of RGC morphology and apoptosis in clinical practice, the relationship between optic nerve structure and visual function cannot be determined intuitively; therefore, further research is needed.
Conflict of Interest None declared
Author contribution Yuan Jiajia: topic selection, research implementation, data collection, data analysis/interpretation, and article drafting. Yong Zhang and Changzheng Chen: topic selection, research implementation, and data collection. Yang Xueying and Miao Qingmei: research implementation, data collection, and data analysis/interpretation. Kai Yoon Fan: statistical analysis and article revision. Li Bin: topic selection and experimental design, article review, and article revision and finalization.
References
[1] Filatov A, Khanni JL, Espinosa PS. Leber hereditary optic neuropathy: case report and literature review[J/OL]. Cureus, 2020, 12(4):e7745[2022-04-10]. https://pubmed.ncbi.nlm.nih.gov/32454526/. DOI: 10.7759/cureus.7745.
[2] Uittenbogaard M, Brantner CA, Fang Z, et al. The m.11778 A > G variant associated with the coexistence of Leber’s hereditary optic neuropathy and multiple sclerosis-like illness dysregulates the metabolic interplay between mitochondrial oxidative phosphorylation and glycolysis[J]. Mitochondrion, 2019, 46:187-194. DOI: 10.1016/j.mito.2018.06.001.
[3] Rovcanin B, Jancic J, Pajic J, et al. Oxidative stress profile in genetically confirmed cases of Leber’s hereditary optic neuropathy[J]. J Mol Neurosci, 2021, 71(5):1070-1081. DOI: 10.1007/s12031-020-01729-y.
[4] Jankauskaitė E, Ambroziak AM, Hajieva P, et al. Testosterone increases apoptotic cell death and decreases mitophagy in Leber’s hereditary optic neuropathy cells[J]. J Appl Genet, 2020, 61(2):195-203. DOI: 10.1007/s13353-020-00550-y.
[5] Marotta R, Chin J, Chiotis M, et al. Long-term screening for primary mitochondrial DNA variants associated with Leber hereditary optic neuropathy: incidence, penetrance and clinical features[J]. Mitochondrion, 2020, 54:128-132. DOI: 10.1016/j.mito.2020.08.007.
[6] Wang D, Liu HL, Du YY, et al. Characterisation of thickness changes in the peripapillary retinal nerve fibre layer in patients with Leber’s hereditary optic neuropathy[J]. Br J Ophthalmol, 2021, 105(8):1166-1171. DOI: 10.1136/bjophthalmol-2020-316573.
[7] Carelli V, Carbonelli M, de Coo IF, et al. International consensus statement on the clinical and therapeutic management of Leber hereditary optic neuropathy[J]. J Neuroophthalmol, 2017, 37(4):371-381. DOI: 10.1097/WNO.0000000000000570.
[8] Karaarslan C. Leber’s hereditary optic neuropathy as a promising disease for gene therapy development[J]. Adv Ther, 2019, 36(12):3299-3307. DOI: 10.1007/s12325-019-01113-2.
[9] Jurkute N, Harvey J, Yu-Wai-Man P. Treatment strategies for Leber hereditary optic neuropathy[J]. Curr Opin Neurol, 2019, 32(1):99-104. DOI: 10.1097/WCO.0000000000000646.
[10] Zuccarelli M, Vella-Szijj J, Serracino-Inglott A, et al. Treatment of Leber’s hereditary optic neuropathy: an overview of recent developments[J]. Eur J Ophthalmol, 2020, 30(6):1220-1227. DOI: 10.1177/1120672120936592.
[11] Zhang Y, Li X, Yuan J, et al. Prognostic factors for visual acuity in patients with Leber’s hereditary optic neuropathy after rAAV2-ND4 gene therapy[J]. Clin Exp Ophthalmol, 2019, 47(6):774-778. DOI: 10.1111/ceo.13515.
[12] Liu HL, Yuan JJ, Zhang Y, et al. Factors associated with rapid improvement in visual acuity in patients with Leber’s hereditary optic neuropathy after gene therapy[J/OL]. Acta Ophthalmol, 2020, 98(6):e730-e733[2022-04-10]. https://pubmed.ncbi.nlm.nih.gov/32096343/. DOI: 10.1111/aos.14379.
[13] Li X, Tian Z, Chen Z, et al. Efficacy evaluation of intravitreal injection of rAAV2-ND4 gene for Leber hereditary optic neuropathy[J]. Chin J Exp Ophthalmol, 2021, 39(8):724-728. DOI: 10.3760/cma.j.cn115989-20210330- 00218.
[14] Yuan JJ, Zhang Y, Wang LL, et al. Visual field variability after gene therapy for Leber’s hereditary optic neuropathy[J]. Ophthalmic Res, 2018, 60(3):176-184. DOI: 10.1159/000487485.
[15] Wan X, Pei H, Zhao MJ, et al. Efficacy and safety of rAAV2-ND4 treatment for Leber’s hereditary optic neuropathy[J/OL]. Sci Rep, 2016, 6:21587[2022-04-11]. https://pubmed.ncbi.nlm.nih.gov/26892229/. DOI: 10.1038/srep21587.
[16] Yang S, Ma SQ, Wan X, et al. Long-term outcomes of gene therapy for the treatment of Leber’s hereditary optic neuropathy[J]. EBioMedicine, 2016, 10:258-268. DOI: 10.1016/j.ebiom.2016.07.002.
[17] Yuan J, Zhang Y, Liu H, et al. Seven-year follow-up of gene therapy for Leber’s hereditary optic neuropathy[J]. Ophthalmology, 2020, 127(8):1125-1127. DOI: 10.1016/j.ophtha.2020.02.023.
[18] Ran R, Yang S, He H, et al. A retrospective analysis of characteristics of visual field damage in patients with Leber’s hereditary optic neuropathy[J/OL]. Springerplus, 2016, 5(1):843[2022-04-12]. https://pubmed.ncbi.nlm.nih.gov/27386292/. DOI: 10.1186/s40064-016-2540-7.