Abstract [View PDF] [Read Full Text]
Objective
To establish an in vitro capsular bag model and compare the inhibitory effects of different 360° square-edge intraocular lens (IOL) on lens epithelial cells (LECs) migration.
Methods
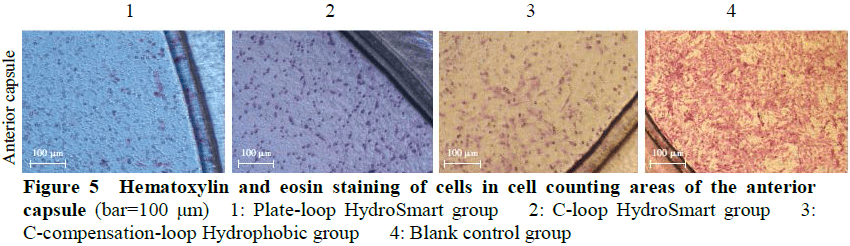
In vitro capsular bag model with posterior capsule opacification (PCO) was established using Transwell compartment, cell climbing slices, human collagen type Ⅳ, and IOL.The models were divided into Plate-loop HydroSmart group, C-loop HydroSmart group, and C-compensation-loop Hydrophobic group according to the different square-edge IOL implanted.A blank control group was set using the Transwell compartment without IOL.The early PCO pathological manifestations in lens epithelial cell line SRA01/04 cultured in the Transwell compartment were observed with an inverted microscope.The cell morphology in different groups was observed by hematoxylin and eosin staining.The cell counting and cell migration inhibition rate of anterior capsule and posterior capsule were calculated by Transwell assay and cell-exclusion zone assay, respectively.
Results
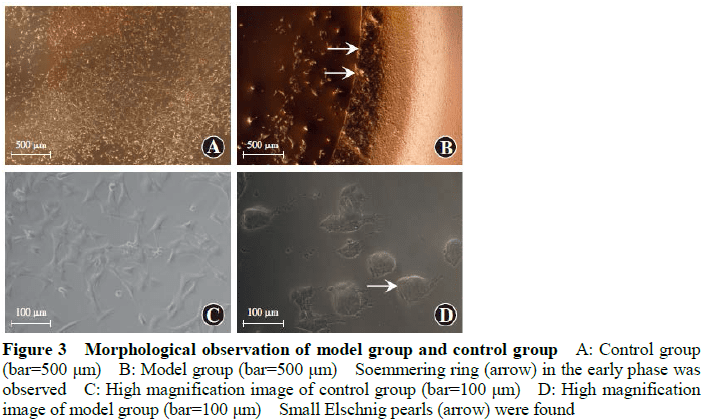
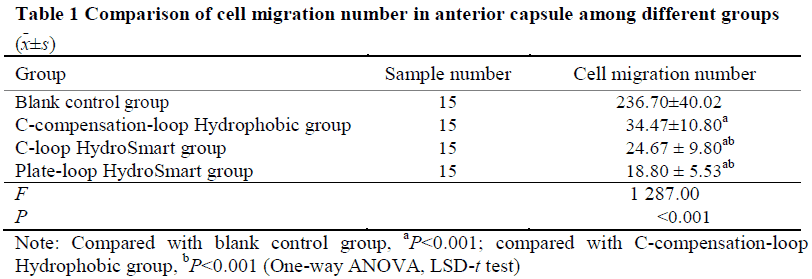
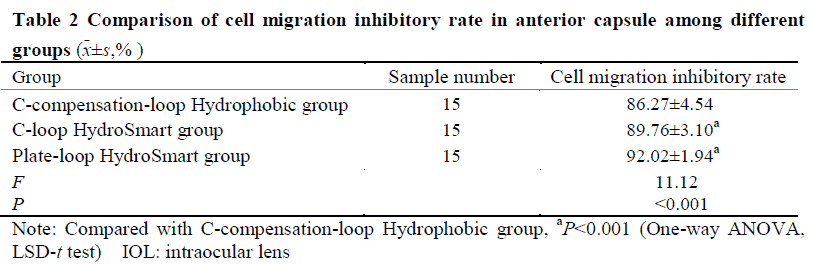
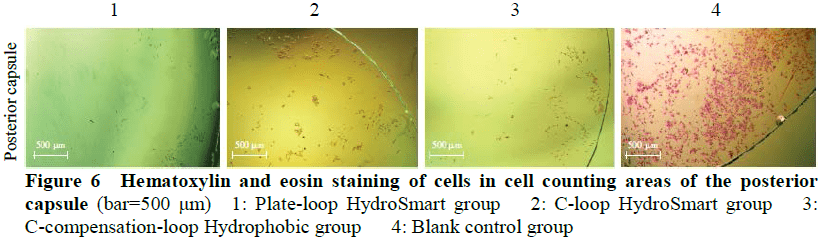
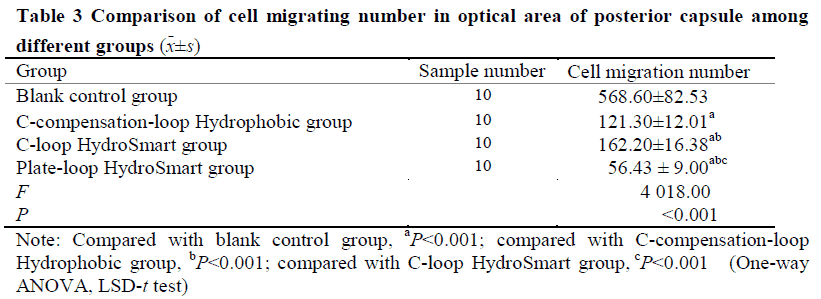
The early pathological characteristics of PCO, such as early Soemmering ring and small Elschnig pearl, could be found in cells in the in vitro capsular bag model after 48-hour culture.The migrating cells in model groups were fibrous.No changes mentioned above were found in blank control group.The number of migrating cells in the anterior capsule of Plate-loop HydroSmart group, C-loop HydroSmart group, C-compensation-loop Hydrophobic group was 18.80±5.53, 24.67±9.80, and 34.47±10.80, respectively, and the number of migrating cells in the optical area of the posterior capsule of the three groups was 56.43±9.00, 162.20±16.38, and 121.30±12.01, respectively.The cell migration inhibition rate in the anterior capsule of Plate-loop HydroSmart group, C-loop HydroSmart group, C-compensation-loop Hydrophobic group was (92.02±1.94)%, (89.76±3.10)%, (86.27±4.54)%, respectively, and the cell migration inhibition rate in optical area of the posterior capsule of the three groups was (91.60±3.65)%, (70.14±5.35)%, (78.43±3.48)%, respectively.The number of migrating cells in the anterior capsule was lower and the cell migration rate inhibition was higher in Plate-loop HydroSmart group than C-compensation-loop Hydrophobic group, with significant differences (both at P<0.05). The number of migrating cells in the optical area of the posterior capsule and the cell migration inhibition rate was greater than those of C-loop HydroSmart group and C-compensation-loop Hydrophobic group, showing statistically significant differences (all at P<0.001).
Conclusions
The in vitro capsular bag model can be used in PCO research.Compared with C-loop HydroSmart IOL and C-compensation-loop Hydrophobic IOL, Plate-loop HydroSmart IOL can more effectively inhibit the migration of LECs to the optical area of the posterior capsule.
Key words:
Figures and tables:





Contributor Information
Department of Ophthalmology, the Fourth Affiliated Hospital of China Medical University, Key Lens Research Laboratory of Liaoning Province, Shenyang 110005, China
Department of Ophthalmology, the Fourth Affiliated Hospital of China Medical University, Key Lens Research Laboratory of Liaoning Province, Shenyang 110005, China
Department of Ophthalmology, the Fourth Affiliated Hospital of China Medical University, Key Lens Research Laboratory of Liaoning Province, Shenyang 110005, China
Department of Ophthalmology, the Fourth Affiliated Hospital of China Medical University, Key Lens Research Laboratory of Liaoning Province, Shenyang 110005, China
Department of Ophthalmology, the Fourth Affiliated Hospital of China Medical University, Key Lens Research Laboratory of Liaoning Province, Shenyang 110005, China
Department of Ophthalmology, the Fourth Affiliated Hospital of China Medical University, Key Lens Research Laboratory of Liaoning Province, Shenyang 110005, China
Department of Ophthalmology, the Fourth Affiliated Hospital of China Medical University, Key Lens Research Laboratory of Liaoning Province, Shenyang 110005, China
Department of Ophthalmology, the Fourth Affiliated Hospital of China Medical University, Key Lens Research Laboratory of Liaoning Province, Shenyang 110005, China