·Clinical Research·
Clinical staging and prognostic risk factors for ocular adnexal lymphoma
Jian Tianming1, Gao Fei1, Yang Wanchen2, Tang Dongrun1, He Yanjin1, Sun Fengyuan1
1Tianjin Key Laboratory of Retinal Functions and Diseases, Tianjin Branch of National Clinical Research Center for Ocular Disease, Eye Institute and School of Optometry, Tianjin Medical University Eye Hospital, Tianjin 300384, China; 2Department of Ophthalmology, the First Hospital of Qinhuangdao, Qinhuangdao 066000, China
Corresponding author: Sun Fengyuan, Email: eyesunfy@126.com
[Abstract] [View PDF in English] [View PDF in Chinese] [Read Full Text]
Objective To evaluate clinical staging and prognostic risk factors for ocular adnexal lymphoma (OAL).
Methods An ambispective cohort study was conducted. Seventy-four patients diagnosed with primary OAL by pathology at Tianjin Medical University Eye Hospital from November 2010 to December 2018 were enrolled. TNM staging of tumor was performed according to local tumor extent and lymph node or systemic involvement. Ann Arbor staging was carried out according to lymph node involvement and extranodal extension. The pathological type was classified according to the World Health Organization classification of lymphoma. The outcome of disease progression or death was analyzed. The Kaplan-Meier method was used for univariate survival analysis and the Cox proportional hazard model was employed for multivariate survival analysis to predict the risk factors affecting prognosis; hazard ratio (HR) and 95% confidence interval were estimated. This study adhered to the Declaration of Helsinki and the study protocol was approved by an Ethics Committee of Tianjin Medical University Eye Hospital (No. 2021KY[L]-32). Written informed consent was obtained from all patients before entering the cohort.
Results For TNM staging, 68 cases were stage < T4 (91.9%), six were stage T4 (8.1%), 71 were stage N0 (95.9%), three were stage ≥N1 (4.1%), and none were stage M. For Ann Arbor staging, 72 cases were stage ⅠE (97.3%) and two were stage ⅡE (2.7%). As for pathological classification, 64 cases were mucosa-associated lymphoid tissue (MALT) lymphoma (86.5%) and 10 were non-MALT lymphoma (13.5%). Follow-up lasted 3–117 months (median, 53 months). Six patients died of the disease and 19 experienced disease progression. The 3-year and 5-year overall survival rate was 96.6% and 86.6%, respectively. The 3-year and 5-year progression-free survival rate was 75.6% and 65.9%, respectively. T4 stage, non-MALT type tumor, and Ki67-positive rate ≥10% were related to declined overall survival rate (P<0.05). T4 stage, ≥N1 stage, ≥Ann Arbor Ⅱ stage, non-MALT type tumor, and Ki67 positive rate ≥10% were related to declined progression-free survival rate (P<0.05). Pathological type (HR=33.193, P=0.003) was an independent risk factor for overall survival rate. N stage (HR=11.683, P=0.001) and pathological type (HR=11.337, P<0.001) were independent risk factors for progression-free survival rate.
Conclusions TNM staging and pathological type are important clinical prognostic indicators for ocular adnexal lymphoma. Patients with high TNM stage or non-MALT lymphoma should be monitored closely.
[Key words] Lymphoma; Prognosis; Survival analysis; Ocular adnexa; Risk factors; TNM classification; Ann Arbor classification
Fund program: The Science & Technology Development Fund of Tianjin Education Commission for Higher Education (2020KJ180)
DOI: 10.3760/cma.j.cn115989-20211231-00727
Ocular adnexal lymphoma (OAL) originates in the conjunctiva, eyelids, lacrimal gland, lacrimal drainage apparatus, and other orbital tissues[1]. The anatomic site and clinical manifestations in OAL are specific and different from systemic lymphomas, which originate in lymph nodes or extranodal organs. Most OALs invade eyelid swelling. Some cases are closely related to systemic lymphomas, with lymph nodes or distant metastasis. Therefore, clinical staging of OAL should be performed precisely according to the extent of tumor invasion, and the use of Ann Arbor and TNM staging systems has become widespread. The Ann Arbor system is mainly used for the clinical staging and treatment of Hodgkin lymphoma, but its prognostic value for OAL remains controversial 2 because OALs are primarily extranodal non-Hodgkin lymphoma. The TNM staging system was initiated by the American Joint Committee on Cancer (AJCC) in the 7th edition of the Cancer Staging Manual and was revised in the 8th edition to assess the prognostic value 3-4. At present, there is no relevant report regarding the effect of TNM staging on the survival and prognosis of OAL in China. In addition, the various pathological types of lymphoma are complex and diverse. The pathological classification of OAL according to the World Health Organization (WHO) classification criteria is helpful for diagnosis and treatment, and is closely related to prognosis 5. Therefore, this retrospective study aimed to evaluate clinical stage and other risk factors in the prognosis of OAL.
1 Methods
1.1 Study design and patients
An ambispective cohort study was conducted. The study was approved by Tianjin Medical University Eye Hospital Foundation Institutional Review Board and adhered to the principles of the Declaration of Helsinki. Informed consent was obtained from all included subjects. A retrospective analysis was performed on the clinical data of patients diagnosed with primary OAL by pathological examination in Tianjin Medical University Eye Hospital (Tianjin, China) from November 2010 to December 2018. Inclusion criteria were as follows: 1) primary OAL; 2) confirmed pathological diagnosis; and 3) follow-up time ≥3 months. Exclusion criteria were as follows: 1) secondary to systemic lymphoma; 2) unclear pathological diagnosis; or 3) incomplete follow-up data or follow-up time < 3 months. A total of 74 cases were ultimately included in the analysis; this included 50 males and 24 females, with a mean age of 64 ± 14 years, and a median medical history of 6 (4, 18) months. A total of 65 cases occurred unilaterally whereas nine occurred bilaterally.
1.2 Clinical stage and pathological classification
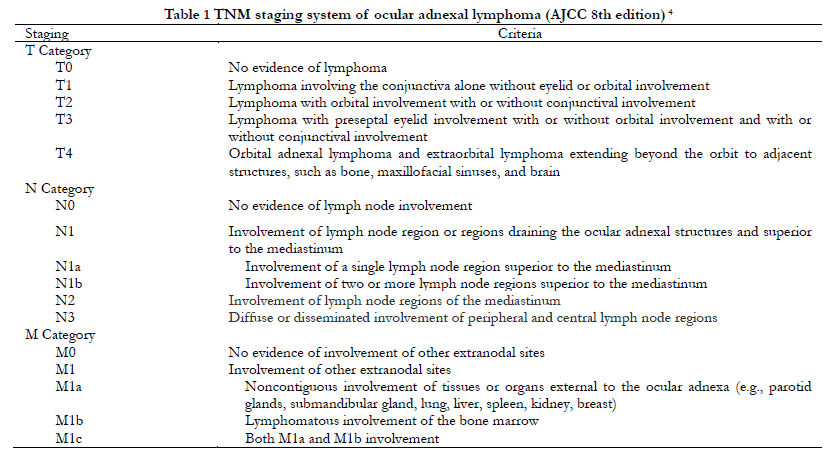
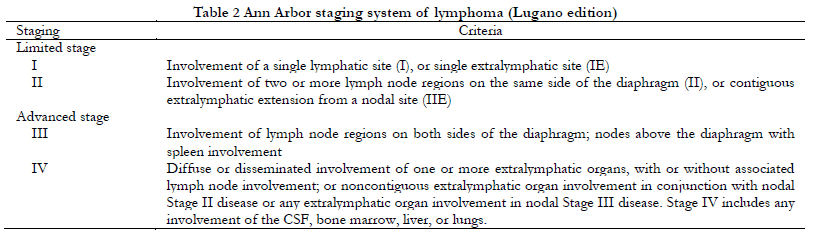
All patients underwent a computed tomography/magnetic resonance imaging (CT/MRI) examination of the orbit, head and neck, chest, and abdomen. TNM staging was performed according to AJCC eighth edition criteria (Table 1), and Ann Arbor staging was performed according to the Lugano revision (Table 2). For bilateral tumors with different T staging, staging for the more severe side was recorded. Pathological diagnosis and classification were made by pathologists from Tianjin Medical University Eye Hospital according to WHO classification criteria 6. The positive rate of Ki-67 was recorded as the percentage of positive immunohistochemical nuclear staining in all tumor cells.
1.3 Treatment method
Treatment protocols included: 1) surgery: complete or mostly complete resection; 2) radiotherapy: conventional fractionated external radiation with total dose 20–36 Gy in 10–18 fractions; 3) chemotherapy: CHOP regimen (cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2, vincristine 1.4 mg/m2, prednisone 40 mg) or R-chop regimen (CHOP regimen plus rituximab 375 mg/m2) every 3 weeks, for a total of 4–6 courses.
1.4 Follow-up and evaluation
The follow-up ended on November 1, 2021. The outcome events included: 1) death from the disease; and (2) progressive disease (PD): any new lesion or increase by ≥50% of previously involved lesions from nadir, respectively 7. Overall survival (OS) and progression-free survival (PFS) were calculated from the date of diagnosis. OS was defined as the period until the date of death or last follow-up, and PFS was defined as the period after the initial treatment until disease progression or until the last follow-up date.
1.5 Statistical analysis
The prognostic factors for OAL were analyzed using SPSS Statistics 25 (NY, USA). Continuous variables were expressed as ±s or median (interquartile range) ([M(Q1, Q3)), according to the normality tests. Categorical variables were expressed as number (percentage). Categorical variables were compared using the χ2 test or Fisher exact test. The one-way ANOVA or Kruskal-Wallis H test, where appropriate, was used to compare continuous variables between two groups. The Kaplan-Meier test was used for univariate survival analysis. The Log-rank test was used to compare survival results of each subgroup. The variables in univariate analysis at a significant level of P<0.1 were then analyzed in a Cox proportional risk model for multivariate survival analysis. Finally, variables were defined as significant at P<0.05.
2 Results
2.1 Comparison of different clinical stages and baseline data
TNM classifications included 11 cases of T1N0, 46 of T2 (45 cases of T2N0 and one of T2N1), 11 of T3N0, six of T4 (four cases of T4N0, one of T4N1, and one of T4N2), and none of M stage. Clinical stage was lower than T4 stage disease for 68 cases (91.9%) and T4 stage disease for six (8.1%). A total of 71 cases (95.9%) were at N0 stage and three (4.1%) were ≥N1 stage. Using the Ann Arbor stage system, 72 cases (97.3%) were IE stage and two (2.7%) were ⅡE stage. There were no significant differences with respect to gender, age, laterality, or medical history between different T stages (P>0.05) (Table 3).
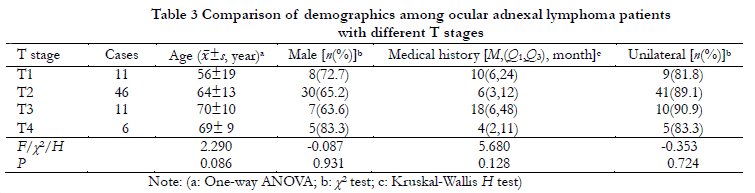
2.2 Comparison different clinical stages and pathological types
Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT) accounted for 64 cases (86.5%); non-MALT lymphoma accounted for 10 cases (13.5%), including diffuse large B-cell lymphoma (DLBCL) in six cases, mantle cell lymphoma (MCL) in two cases, and T-cell lymphoma (TCL) in two cases. The Ki67-positive rate for MALT lymphoma was 5% (1%, 6%), which was significantly lower than that of non-MALT lymphoma [55% (9%, 80%)], and this difference was statistically significant (P<0.05). The proportion of MALT lymphoma <T4 stage was higher than that of non-MALT lymphoma, and this difference was statistically significant (P=0.002). There were no significant differences in N stage or Ann Arbor stage distribution among different pathological types (P>0.05) (Table 4).

2.3 Comparison of cases by treatment type, clinical stage, and pathological type
All patients were treated with surgery, followed by postoperative radiotherapy in 24 cases (mean radiation dose 33 Gy) or chemotherapy in 25 cases (CHOP in 19 cases, R-CHOP in six cases). The treatment group was divided into a surgery group (n=34, 45.9%), surgery+radiotherapy group (n=15, 20.3%), surgery+chemotherapy group (n=16, 21.6%), and surgery+radiotherapy+chemotherapy group (n=9, 12.2%). There were no significant differences with respect to pathological type or T, N, and Ann Arbor stage among different treatment groups (P>0.05) (Table 5).

2.4 Survival analysis
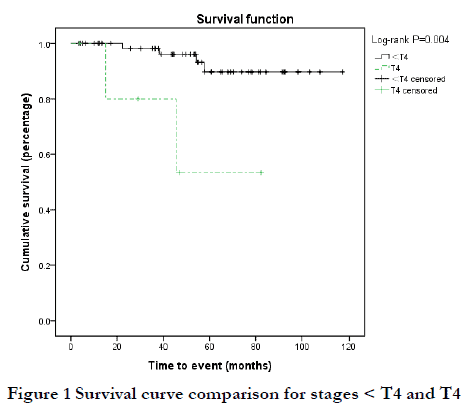
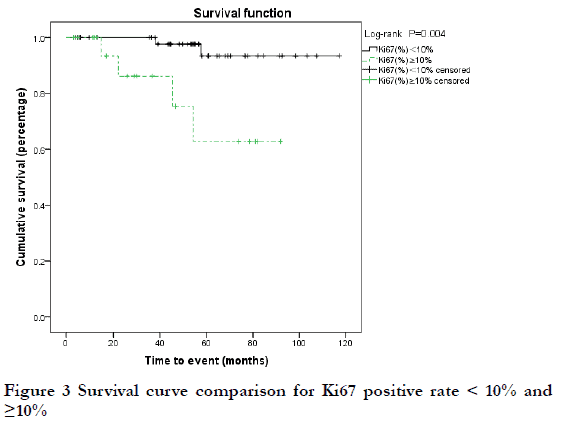
The follow-up time of 74 patients ranged from 3 to 117 months, with a median of 53 months. Six patients (8.1%) died of the disease (DOD; extensive tumor metastasis or organ failure), and the 3-year and 5-year overall survival (OS) rates were 96.6% and 86.6%, respectively. Among the six DOD cases, there were two cases each of T2N0, T3N0, and T4N0 stage; six cases of Ann Arbor I stage; and three cases each of MALT and non-MALT. By Kaplan-Meier analysis, the 5-year OS rate was 53.3% for T4 stage disease and 89.7% for < T4 stage disease was; this difference was statistically significant (P<0.05) (Figure 1). The 3-year OS rate of MALT and non-MALT disease was 100% and 71.4%, respectively, and this difference was statistically significant (P<0.05) (Figure 2). High Ki67-positive rate may be a risk factor for death, and the data was grouped by < and ≥third quartile (Q3 = 10%). The 5-year OS rate of Ki67 (%) < 10% and ≥10% was 93.4% and 62.8%, respectively, and this difference was statistically significant (P<0.05) (Figure 3). Age may be a factor affecting OS, and the data were grouped according to Q3 = 72 years. The 5-year OS of age < 72 years and ≥72 years was 92.1% and 76.3%, respectively, and there was no significant difference between these two groups (P=0.09) (Figure 4). There were no significant differences in OS with respect to sex, medical history, laterality, N stage, Ann Arbor stage, or treatment group (P>0.1).


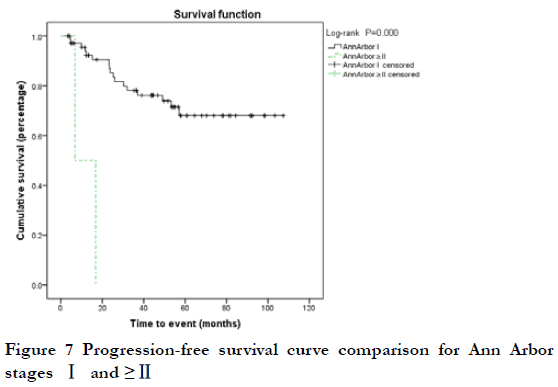
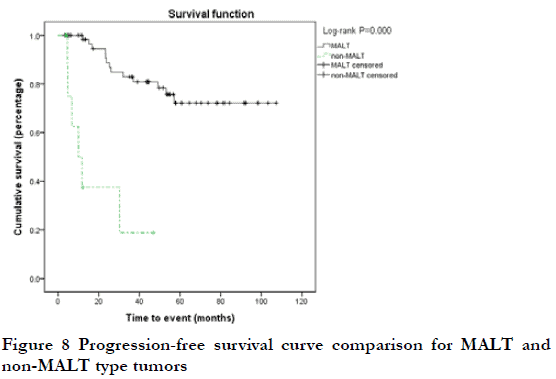
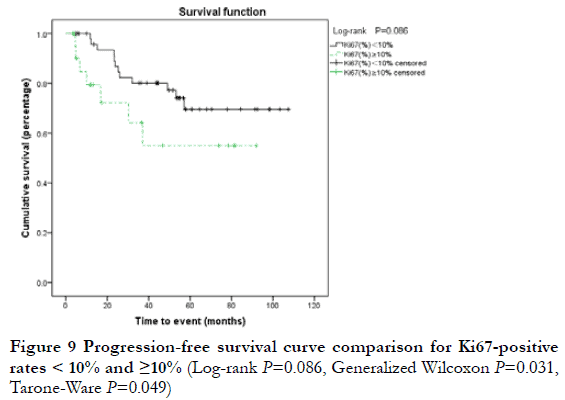
At the end of the follow-up period, 19 patients (25.7%) were considered to have progressive disease (PD) (including 12 cases of local relapse and eight cases of lymph node metastasis or systemic metastasis); the 3-year and 5-year PFS rates were 75.6% and 65.9%, respectively. Among the 19 cases of PD, two were T1N0, nine were T2N0, one was T2N1, three were T3N0, two were T4N0, one was T4N1, and one was T4N2; 17 cases were Ann Arbor I stage and two were ≥II stage; 13 cases were MALT and six were non-MALT type tumors. By Kaplan-Meier analysis, the 3-year PFS rate of T4 and < T4 stage was 20.0% and 80.6%, respectively; this difference was statistically significant (P<0.05) (Figure 5). The 3-year PFS rate of N0, ≥N1, Ann Arbor I, and ≥II groups was 79.6%, 78.1%, 0, and 0, respectively; there were statistically significant differences between N0 and ≥N1 stage, and between Ann Arbor I and ≥II stage (P<0.05) (Figure 6-7). The 3-year PFS rate of MALT and non-MALT type tumors was 82.9% and 18.8%, respectively, with statistically significant differences between the two groups (P<0.05) (Figure 8). The 5-year PFS rate of Ki67 (%) < and ≥10% groups was 69.5% and 55.0%, respectively. PFS curves showed statistically significant differences between the two groups in the early stage (P<0.05), but not in the late stage (P>0.05) (Figure 9). The 3-year PFS rate of the unilateral ocular involvement group was 81.5%, and that of the bilateral group was 31.3%; the difference between two groups was statistically significant (P<0.05) (Figure 10). There were no significant differences in PFS with respect to different gender, age, medical history, or treatment group (P>0.05).



2.5 Prognostic factors
The variables showing P<0.1 in the univariate analysis (T stage, pathological type, Ki67-positive rate. and age) were analyzed by Cox multivariate regression. Pathological type was found to be an independent risk factor affecting OS (HR=33.193, P=0.003). T stage, N stage, Ann Arbor stage, pathological type, and Ki67-positive rate were analyzed by Cox multivariate regression. The results showed that N stage (HR=11.683, P=0.001) and pathological type (HR=11.337, P=0.000) were independent risk factors for PFS (Table 6−7).


3 Discussion
Ann Arbor staging was originally designed for Hodgkin lymphoma, and includes nodal and extranodal involvement as well as the presence or absence of systemic symptoms. In the Lugano revision, the scope of extranodal sites was revised to emphasize the continuous or discontinuous trend of extranodal extension, such as retaining stage I and II with extranodal involvement, removing the substages of III with extranodal involvement, upgrading stage II with discontinuous extranodal organ involvement, and stage III with any extranodal organ involvement to stage IV 7. OAL, as a primary conjunctival, orbital, or palpebral tumor with adjacent lymph node involvement, normally belongs to Ann Arbor stage I or II. Stage III–IV disease and systemic symptoms have been associated with poor prognosis 8. In this study, Ann Arbor staging was concentrated on stage IE, which was not conducive to substage survival analysis. For lymphoma specifically localized to the ocular adnexal region, another staging system is needed to assess the tumor invasion.
The TNM staging system was revised in the AJCC 8th edition by deleting the substages of T1, T2, and T4 from T stage, as well as redefining N stage by the number of lymph nodes involved in the ocular adnexal drainage area and the position of mediastinum. Multiple studies suggest that the TNM system is useful for disease prognosis, but the conclusions of prognostic values show some differences. Sniegowski et al. 9 reported that increased T stage was related to decreased disease-free survival, and the OS rate of T4 stage tumors was significantly reduced. Nam et al. 10 proposed that the complete response rate of lymphoma located in the conjunctiva was higher after initial treatment, but there was no difference in recurrence and mortality between conjunctiva and non-conjunctiva groups. Kwon 11 used the AJCC 8th edition stage system for OAL to perform univariable analyses, and found that patients in ≥T2 and ≥N1 stages had increased risk of recurrence and decreased PFS, among which T4 stage was closely related to distant relapse (HR=11.08). Our study aimed to validate the new AJCC staging system to determine the prognosis of patients with OAL at univariate and multifactorial levels, and found that the risk of death in T4 stage patients was eight times higher than that in < T4 stage patients. Furthermore, we found that the risk of progressive disease in T4 and ≥N1 cases was six and twelve times higher, respectively, than that in < T4 and N0 cases. By multiple factors, T stage was not an independent risk factor for reducing OS or PFS, but ≥N1 stage and non-MALT pathological type did show significant regulatory effects on disease progression. These results suggest that high T category is predictive of decreased OS or DFS, which may be affected by the synergistic effect of N stage and pathological type. If a T4 tumor spreads out of the orbit, it will invade the paranasal sinus, temporal fossa, skull base, and other sites, and can spread more easily through the lymphatic pathway to result in metastasis or death. While low T category may not be significantly associated with recurrence or survival, it is more meaningful for accurately documenting lesions during follow up.
Pathological type is an important factor affecting the prognosis of disease. The most common pathological type is MALT lymphoma, followed by follicular lymphoma (FL), diffuse large B-cell lymphoma (DLBCL), mantle cell lymphoma (MCL), as well as others 12. MALT and FL tumors usually present with local indolent growth. Their prognoses (10-year OS rate 92% and 71%, respectively) are significantly better than DLBCL and MCL (10-year OS rate 41% and 32%, respectively), and the risk of reduced OS and PFS about 25 times and six times that of DLBCL and MCL, respectively 11,13. Some studies have pointed out that DLBCL is an independent risk factor for disease recurrence in common pathological types 14. However, due to the limitation of samples, the proportion of some pathological types in this study was small or missing. We divided the patients into two groups, and found that the risk of death and disease progression in non-MALT lymphoma was about 33 and 11 times greater, respectively, than with MALT lymphoma. High-grade lymphomas such as DLBCL, MCL, and nature killer (NK)/T are characterized by aggressiveness, accompanied by a large number of mitotic figures and a high proliferation index, which are of significant prognostic value 15. Ki67 is a monoclonal antibody combined with proliferating cell nuclear antigen. The Ki67 proliferation index can be used to reflect the characteristics of tumor cell proliferation and to predict clinical outcomes. This should be assessed by counting the percentage of cells with positive nuclear expression of Ki67, with a Ki67 (%) > 30% usually indicating a poor clinical prognosis 16. In this study, the risk of death in the Ki67 (%) ≥10% group was approximately eight times higher than that in the Ki67 (%) < 10% group. Considering the median Ki67 (%) of non-MALT tumors was 55%, which was significantly higher than that of MALT type tumors, it was speculated that high Ki67 index and high-grade pathological type might have a synergistic effect. However, at the multifactorial level, pathology was the only risk factor affecting OS and PFS. Therefore, Ki67 (%) indicated tumor proliferation activity and risk of early recurrence, but did not independently regulate OS or PFS under the influence of pathological types and other factors.
MALT lymphoma is sensitive to radiotherapy, with the 5-year local control rate reaching 100% at an average dose of 30 Gy, the 5-year OS ranging from 89% to 100%, and the 5-year PFS ranging from 75% to 92% 17-22. Oh et al. 23 reported that radiotherapy also had a good effect on bilateral disease, and bilateral low-dose radiotherapy (< 30 Gy) had a tendency to prolong PFS more than chemotherapy. Treatment of DLBCL has not yet formed into a unified plan, and a combination treatment of surgery, chemotherapy, and radiotherapy is mostly used with a 5-year OS of 20% to 36% 24-25. At present, immunochemotherapy using a B cell monoclonal antibody (Rituximab) is the standard treatment for B-cell derived lymphoma, and more rigorous controlled clinical studies are needed 26. Nowakowski et al. 27 applied R‑CHOP in combination with Lenalidomide in the treatment of Ann Arbor II-IV stage DLBCL with an overall response rate of 98%, a complete response rate of 80%, and 2-year PFS and OS of 59% and 78%, respectively. Kim et al. 28 applied an R-CVP (rituximab, cyclophosphamide, vincristine, and prednisolone) regimen for bilateral or extra-conjunctival involved ocular MALT lymphoma, and reported a 4-year PFS and OS of 90.3% and 100%, respectively. The results of this analysis showed that there was no statistical difference in PFS or OS among all treatment regimens. In consideration of age, pathological type, chemotherapy side effects, and other factors, postoperative radiotherapy should be the first choice and follow-up protocols for high-risk patients should be strengthened. Due to the limited sample size of this study, further large-sample, multi-center clinical studies are needed to evaluate the synergistic and overall effects of related factors on OAL disease prognosis.
In conclusion, TNM stage and pathological type are important clinical prognostic indicators of ocular adnexal lymphoma. Under the effect of a single factor, the risk of death and disease progression was higher in patients with T4 stage, ≥N1 stage, and non-MALT tumors. Under the effect of multiple factors, pathological type was an independent risk factor of OS and PFS, and N stage was an independent risk factor of PFS. Patients with high TNM stage or non-MALT type tumors should be monitored closely.
Conflict of interest None declared.
Author contributions Jian Tianming: conceived and designed the research, collected the data, performed the analysis, wrote the paper; Gao Fei, Yang Wanchen: collected the data, revise the paper; Tang Dongrun, He Yanjin: collect the cases, performed the analysis; Sun Fengyuan: conceived and designed the research, collected the data, revise the paper
References
[1] Olsen TG, Heegaard S. Orbital lymphoma[J]. Surv Ophthalmol, 2019, 64(1):45-66. DOI: 10.1016/j.survophthal.2018.08.002.
[2] Carbone PP, Kaplan HS, Musshoff K, et al. Report of the committee on Hodgkin’s disease staging classification[J]. Cancer Res, 1971, 31(11):1860-1861.
[3] Finger PT, 7th Edition, AJCC-UICC Ophthalmic Oncology Task Force. The 7th edition AJCC staging system for eye cancer: an international language for ophthalmic oncology[J]. Arch Pathol Lab Med, 2009, 133(8):1197-1198. DOI: 10.5858/133.8.1197.
[4] Amin MB, Edge SB, Greene FL, et al. AJCC cancer staging manual[M]. 8th ed. Chicago: American college of surgeons. 2017: 849-854.
[5] Cho EY, Han JJ, Ree HJ, et al. Clinicopathologic analysis of ocular adnexal lymphomas: extranodal marginal zone b-cell lymphoma constitutes the vast majority of ocular lymphomas among Koreans and affects younger patients[J]. Am J Hematol, 2003, 73(2):87-96. DOI: 10.1002/ajh.10332.
[6] Swerdlow SH, Campo E, Harris NL, et al. World health organization classification of tumours of haematopoietic and lymphoid tissues[M]. 4th ed. Lyon: IARC, 2008:109-138.
[7] Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification[J]. J Clin Oncol, 2014, 32(27):3059-3068. DOI: 10.1200/JCO.2013.54.8800.
[8] .Li J, Wang YC, Chen LX, et al. Clinical and pathological analysis of ocular adnexal diffuse large B-cell lymphoma[J]. Chin J Ophthalmol, 2021, 57(5):366-371. DOI: 10.3760/cma.j.cn112142-20200703-00446.
[9] Sniegowski MC, Roberts D, Bakhoum M, et al. Ocular adnexal lymphoma: validation of American Joint Committee on Cancer seventh edition staging guidelines[J]. Br J Ophthalmol, 2014, 98(9):1255-1260. DOI: 10.1136/bjophthalmol-2013-304847.
[10] Nam SW, Woo KI, Kim YD. Characteristics of primary extranodal marginal zone B-cell lymphoma in Korea: conjunctiva versus other ocular adnexa[J]. Br J Ophthalmol, 2018, 102(4):502-508. DOI: 10.1136/bjophthalmol-2017-310741.
[11] Kwon M, Lee JS, Lee C, et al. Prognostic factors for relapse and survival among patients with ocular adnexal lymphoma: validation of the eighth edition of the American Joint Committee on Cancer (AJCC) TNM classification[J]. Br J Ophthalmol, 2021, 105(2):279-284. DOI: 10.1136/bjophthalmol-2020-315875.
[12] Jakobiec FA. Ocular adnexal lymphoid tumors: progress in need of clarification[J]. Am J Ophthalmol, 2008, 145(6):941-950. DOI: 10.1016/j.ajo.2008.03.013.
[13] Olsen TG, Holm F, Mikkelsen LH, et al. Orbital lymphoma-an international multicenter retrospective study[J]. Am J Ophthalmol, 2019, 199:44-57. DOI: 10.1016/j.ajo.2018.11.002.
[14] Savino G, Midena G, Blasi MA, et al. Orbital and eyelid B-cell lymphoma: a multicenter retrospective study[J/OL]. Cancers (Basel), 2020, 12(9):2538 [2021-12-05]. https://pubmed.ncbi.nlm.nih.gov/32906630/. DOI: 10.3390/cancers12092538.
[15] Suzuki R. Pathogenesis and treatment of extranodal natural killer/T-cell lymphoma[J]. Semin Hematol, 2014, 51(1):42-51. DOI: 10.1053/j.seminhematol.2013.11.007.
[16] Schaffel R, Hedvat CV, Teruya-Feldstein J, et al. Prognostic impact of proliferative index determined by quantitative image analysis and the International Prognostic Index in patients with mantle cell lymphoma[J]. Ann Oncol, 2010, 21(1):133-139. DOI: 10.1093/annonc/mdp495.
[17] Son SH, Choi BO, Kim GW, et al. Primary radiation therapy in patients with localized orbital marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT Lymphoma)[J]. Int J Radiat Oncol Biol Phys, 2010, 77(1):86-91. DOI: 10.1016/j.ijrobp.2009.04.018.
[18] Zhao SX, Su D, Xu Y, et al. Clinical efficacy of radiotherapy for stage IE primary ocular adnexal mucosa associated lymphoid tissue lymphoma[J]. Chin J Radiat Oncol, 2019, 28(2):108-112. DOI: 10.3760/cma.j.issn.1004-4221.2019.02.006.
[19] Fung CY, Tarbell NJ, Lucarelli MJ, et al. Ocular adnexal lymphoma: clinical behavior of distinct World Health Organization classification subtypes[J]. Int J Radiat Oncol Biol Phys, 2003, 57(5):1382-1391. DOI: 10.1016/s0360-3016(03)00767-3.
[20] De Cicco L, Cella L, Liuzzi R, et al. Radiation therapy in primary orbital lymphoma: a single institution retrospective analysis[J/OL]. Radiat Oncol, 2009, 4:60[2021-12-06]. https://pubmed.ncbi.nlm.nih.gov/19968864/. DOI:10.1186/ 1748-717X-4-60.
[21] Hashimoto N, Sasaki R, Nishimura H, et al. Long-term outcome and patterns of failure in primary ocular adnexal mucosa-associated lymphoid tissue lymphoma treated with radiotherapy[J]. Int J Radiat Oncol Biol Phys, 2012, 82(4):1509-1514. DOI: 10.1016/j.ijrobp.2011.04.052.
[22] Nam H, Ahn YC, Kim YD, et al. Prognostic significance of anatomic subsites: results of radiation therapy for 66 patients with localized orbital marginal zone B cell lymphoma[J]. Radiother Oncol, 2009, 90(2):236-241. DOI: 10.1016/j.radonc. 2008.09.011.
[23] Oh SY, Kim WS, Kang HJ, et al. Treating synchronous bilateral ocular adnexal marginal zone lymphoma: the consortium for improving survival of lymphoma study[J]. Ann Hematol, 2018, 97(10):1851-1857. DOI: 10.1007/s00277-018-3387-5.
[24] Munch-Petersen HD, Rasmussen PK, Coupland SE, et al. Ocular adnexal diffuse large B-cell lymphoma: a multicenter international study[J]. JAMA Ophthalmol, 2015, 133(2):165-173. DOI: 10.1001/jamaophthalmol.2014.4644.
[25] Rasmussen PK, Ralfkiaer E, Prause JU, et al. Diffuse large B-cell lymphoma of the ocular adnexal region: a nation-based study[J]. Acta Ophthalmol, 2013, 91(2):163-169. DOI: 10.1111/j.1755-3768.2011.02337.x.
[26] Guffey Johnson J, Terpak LA, Margo CE, et al. Extranodal marginal zone B-cell lymphoma of the ocular adnexa[J]. Cancer Control, 2016, 23(2):140-149. DOI: 10.1177/107327481602300208.
[27] Nowakowski GS, LaPlant B, Macon WR, et al. Lenalidomide combined with R-CHOP overcomes negative prognostic impact of non-germinal center B-cell phenotype in newly diagnosed diffuse large B-Cell lymphoma: a phase II study[J]. J Clin Oncol, 2015, 33(3):251-257. DOI: 10.1200/JCO.2014.55.5714.
[28]Kim SY, Yang SW, Lee WS, et al. Frontline treatment with chemoimmunotherapy for limited-stage ocular adnexal MALT lymphoma with adverse factors: a phase II study[J/OL]. Oncotarget, 2017, 8(40):68583-68590[2021-12-08]. https://pubmed.ncbi.nlm.nih.gov/28978139/. DOI: 10.18632/oncotarget.19788.