Authors: Yang Jingjing, Liang Zhen, Lu Ping, Zhang Zhen, Zhang Junjie
Abstract [View PDF] [Read Full Text]
Objective
To prepare vorinostat encapsulated hydroxypropyl-β-cyclodextrin (SAHA-CD) eye drops and investigate its inhibitory effect on corneal neovascularization (CNV) induced by alkali burns in mouse.
Methods
The SAHA-CD eye drops at concentrations of 0.1%, 0.2%and 0.4%were prepared by inclusion technology with hydroxypropyl-β-cyclodextrin, and the content was assayed by high performance liquid chromatography.Seventy-five SPF mice with alkali burn-induced CNV were randomized into 0.1%SAHA-CD group, 0.2%SAHA-CD group, 0.4%SAHA-CD group, dexamethasone group and normal control group according to a random number table, 15 for each group, among which the SAHA-CD groups and dexamethasone group were treated with corresponding drugs, and model control group was treated with normal saline immediately after modeling, four times a day and five microliters each time, lasting for six days.The healing of corneal epithelium was examined with a slit lamp microscope after fluorescein sodium staining, and the areas of cornea epithelial defects were calculated using Eyestudio software.The corneal flat mount was prepared, and the length and areas of CNV were calculated with ImageJ software.The histology of mouse corneas was observed through hematoxylin and eosin staining.The expression level of vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF) and matrix metalloproteinase-9 (MMP-9) in cornea were measured with enzyme linked immunosorbent assay (ELISA) kits.The use and care of animals complied with the ARVO statement and this study protocol was approved by the Experimental Animal Ethics Committee of Henan Eye Institute (No.HNEECA-2020-01).
Results
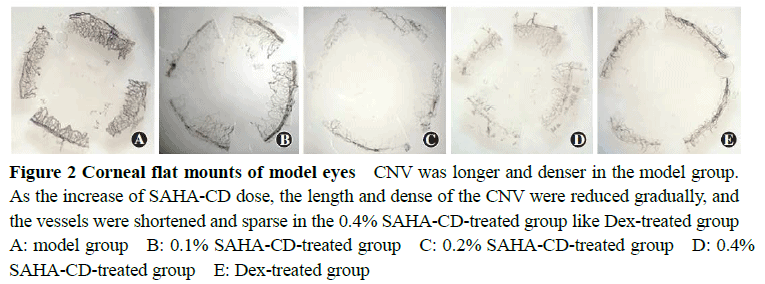
The actual drug contents of the 0.1%, 0.2% and 0.4%SAHA-CD eye drops were 97.62%, 98.33%and 98.14%of the labeled amount.The cornea showed edema and opacification after modeling.On the sixth day after treatment, significant differences were found in the length and areas of CNV among various groups (F=7.655, 8.802; both at P<0.01).The areas of CNV in 0.2%SAHA-CD, 0.4%SAHA-CD and dexamethasone groups were significantly smaller than model control group, and the length of CNV in 0.1%SAHA-CD, 0.2%SAHA-CD and dexamethasone groups were significantly smaller than model control group (all at P<0.05).On the third and sixth day following modeling, significant differences in the expression levels of VEGF, bFGF and MMP-9 were found among the five groups (third day: F=6.345, 7.149, 18.650; all at P<0.01; sixth day: F=6.749, 5.105, 5.023; all at P<0.01), and the expression levels of VEGF, bFGF and MMP-9 in 0.2%SAHA-CD group were significantly lower than those in 0.1%SAHA-CD group, 0.4%SAHA-CD group and model control group (all at P<0.05).
Conclusions
SAHA-CD eye drops can inhibit alkali burn-induced CNV in mouse.
Key words:
Figures and Tables




Contributor Information
Department of Ophthalmology, Henan Provincial People’s Hospital, People’s Hospital of Zhengzhou University, Henan Eye Hospital, Zhengzhou 450003, China
Department of Ophthalmology, Henan Provincial People’s Hospital, People’s Hospital of Zhengzhou University, Henan Eye Hospital, Zhengzhou 450003, China
Department of Ophthalmology, Henan Provincial People’s Hospital, People’s Hospital of Zhengzhou University, Henan Eye Hospital, Zhengzhou 450003, China
Department of Ophthalmology, Henan Provincial People’s Hospital, People’s Hospital of Zhengzhou University, Henan Eye Hospital, Zhengzhou 450003, China
Department of Ophthalmology, Henan Provincial People’s Hospital, People’s Hospital of Zhengzhou University, Henan Eye Hospital, Zhengzhou 450003, China